Related guidance:
Involving people in decision-making
- Encourage and enable people living with dementia to give their own views and opinions about their care.
- If needed, use additional or modified ways of communicating (for example visual aids or simplified text).
- Consider using a structured tool to assess the likes and dislikes, routines and personal history of a person living with dementia.
Providing information
- Provide people living with dementia and their family members or carers (as appropriate) with information that is relevant to their circumstances and the stage of their condition.
See Easy Read dementia factsheets (Alzheimer’s Society)
- Be aware of the obligation to provide accessible information as detailed in the NHS Accessible Information Standard. For more guidance on providing information and discussing people’s preferences with them, see the NICE guidelines on patient experience in adult NHS services and people’s experience in adult social care services.
- At diagnosis, offer the person and their family members or carers (as appropriate) oral and written information that explains:
• what their dementia subtype is and the changes to expect as the condition progresses
• which healthcare professionals and social care teams will be involved in their care and how to contact them
• if appropriate, how dementia affects driving, and that they need to tell the Driver and Vehicle Licensing Agency (DVLA) and their car insurer about their dementia diagnosis
• their legal rights and responsibilities
• their right to reasonable adjustments (in line with the Equality Act 2010) if they are working or looking for work
• how the following groups can help and how to contact them:
- local support groups, online forums and national charities
- financial and legal advice services
- advocacy services.
See Somerset Council Dementia webpage
- If it has not been documented earlier, ask the person at diagnosis:
• for their consent for services to share information
• which people they would like services to share information with (for example family members or carers)
• what information they would like services to share. Document these decisions in the person’s records. - After diagnosis, direct people and their family members or carers (as appropriate) to relevant services for information and support (single named health or social care professional who is responsible for coordinating their care).
- For people who do not want follow-up appointments and who are not using other services, ask if they would like to be contacted again at a specified future date.
- Ensure that people living with dementia and their carers know how to get more information and who from if their needs change.
- Tell people living with dementia (at all stages of the condition) about research studies they could participate in.
Advance care planning
- Offer early and ongoing opportunities for people living with dementia and people involved in their care to discuss:
• the benefits of planning ahead
• lasting power of attorney (for health and welfare decisions and property and financial affairs decisions)
• an advance statement about their wishes, preferences, beliefs and values regarding their future care
• advance decisions to refuse treatment
• their preferences for place of care and place of death.
Explain that they will be given chances to review and change any advance statements and decisions they have made. - At each care review, offer people the chance to review and change any advance statements and decisions they have made.
Initial assessment in non-specialist settings
- At the initial assessment take a history (including cognitive, behavioural and psychological symptoms, and the impact symptoms have on their daily life):
• from the person with suspected dementia and
• if possible, from someone who knows the person well (such as a family member). - If dementia is still suspected after initial assessment:
• conduct a physical examination and
• undertake appropriate blood and urine tests to exclude reversible causes of cognitive decline and
• use cognitive testing. - When using cognitive testing, use a validated brief structured cognitive instrument such as:
• the 10-point cognitive screener (10-CS)
• the 6-item cognitive impairment test (6CIT)
• the 6-item screener
• the Memory Impairment Screen (MIS)
• the Mini-Cog
• Test Your Memory (TYM). - Do not rule out dementia solely because the person has a normal score on a cognitive instrument.
- When taking a history from someone who knows the person with suspected dementia, consider supplementing this with a structured instrument such as the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) or the Functional Activities Questionnaire (FAQ).
- Refer the person to a specialist dementia diagnostic service (such as a memory clinic or community old age psychiatry service) if:
• reversible causes of cognitive decline (including delirium, depression, sensory impairment [such as sight or hearing loss] or cognitive impairment from medicines associated with increased anticholinergic burden) have been
investigated and
• dementia is still suspected. - If the person has suspected rapidly-progressive dementia, refer them to a neurological service with access to tests (including cerebrospinal fluid examination) for Creutzfeldt–Jakob disease and similar conditions.
- For more guidance on assessing for dementia in people with learning disabilities, see the NICE guideline on mental health problems in people with learning disabilities.
Review after diagnosis
- After a person is diagnosed with dementia, ensure they and their family members or carers (as appropriate) have access to a memory service or equivalent hospital- or primary-care-based multidisciplinary dementia service.
- Memory services and equivalent hospital- and primary-care-based multidisciplinary dementia services should offer a choice of flexible access or prescheduled monitoring appointments.
- When people living with dementia or their carers have a primary care appointment, assess for any emerging dementia-related needs and ask them if they need any more support.
Care coordination
- Provide people living with dementia with a single named health or social care professional who is responsible for coordinating their care.
- Named professionals should:
• arrange an initial assessment of the person’s needs, which should be face to face if possible
• provide information about available services and how to access them
• involve the person’s family members or carers (as appropriate) in support and decision-making
• give special consideration to the views of people who do not have capacity to make decisions about their care, in line with the principles of the Mental Capacity Act 2005
• ensure that people are aware of their rights to and the availability of local advocacy services, and if appropriate to the immediate situation an independent mental capacity advocate
• develop a care and support plan, and:
- agree and review it with the involvement of the person, their family members or carers (as appropriate) and relevant professionals
- specify in the plan when and how often it will be reviewed
- evaluate and record progress towards the objectives at each review
- ensure it covers the management of any comorbidities
- provide a copy of the plan to the person and their family members or carers (as appropriate).
Transferring information between services and care settings
- When developing care and support plans and advance care and support plans, request consent to transfer these to different care settings as needed.
- Service providers should ensure that information (such as care and support plans and advance care and support plans) can be easily transferred between different care settings (for example home, inpatient, community and residential care).
- Staff delivering care and support should maximise continuity and consistency of care. Ensure that relevant information is shared and recorded in the person’s care and support plan.
Green Bag Scheme
- The Somerset Green Bag Scheme is now available.
- The purpose of this scheme is to encourage patients going into hospital (particularly as an inpatient or as an emergency) to take all their medications with them in a clearly identifiable bag (ie, the Green Bag) so that the hospital staff are aware of the medications you are taking, and the medications can stay with you if you transfer between wards or care settings.
- Knowing what medications are being taken by the patient helps the medical team in their initial assessment and ensuring patient safety.
- If you feel you are likely to need a hospital admission (either for planned care or are at risk of an unplanned admission due to ongoing health issues) please contact your local GP practice for details.
Making services accessible
- Service providers should design services to be accessible to as many people living with dementia as possible, including:
• people who do not have a carer or whose carer cannot support them on their own
• people who do not have access to affordable transport, or find transport difficult to use
• people who have other responsibilities (such as work, children or being a carer themselves)
• people with learning disabilities, sensory impairment (such as sight or hearing loss) or physical disabilities
• people who may be less likely to access health and social care services, such as people from black, Asian and minority ethnic groups.
Interventions to promote cognition, independence and wellbeing
- Offer a range of activities to promote wellbeing that are tailored to the person’s preferences.
- Offer group cognitive stimulation therapy to people living with mild to moderate dementia.
- Consider group reminiscence therapy for people living with mild to moderate dementia.
- Consider cognitive rehabilitation or occupational therapy to support functional ability in people living with mild to moderate dementia.
- Do not offer acupuncture to treat dementia.
- Do not offer ginseng, vitamin E supplements, or herbal formulations to treat dementia.
- Do not offer cognitive training to treat mild to moderate Alzheimer’s disease.
- Do not offer interpersonal therapy to treat the cognitive symptoms of mild to moderate Alzheimer’s disease.
- Do not offer non-invasive brain stimulation (including transcranial magnetic stimulation) to treat mild to moderate Alzheimer’s disease, except as part of a randomised controlled trial.
Pharmacological interventions for dementia
Managing medicines for all dementia subtypes
- For guidance on managing medicines (including covert administration), see the NICE guidelines on managing medicines for adults receiving social care in the community and managing medicines in care homes.
See Covert administration of medicines in adults: pharmaceutical issues (SPS)
Pharmacological management of Alzheimer’s disease
- The three acetylcholinesterase (AChE) inhibitors donepezil, galantamine and rivastigmine as monotherapies are recommended as options for managing mild to moderate Alzheimer’s disease under all of the conditions specified in below
• For people who are not taking an AChE inhibitor or memantine, prescribers should only start treatment with these on the advice of a clinician who has the necessary knowledge and skills. This could include:
- secondary care medical specialists such as psychiatrists, geriatricians and neurologists
- other healthcare professionals (such as GPs, nurse consultants and advanced nurse practitioners), if they have specialist expertise in diagnosing and treating Alzheimer’s disease.
NHS Somerset Dementia including Alzheimer’s Disease Shared Care Protocol.
- Once a decision has been made to start an AChE inhibitor or memantine, the first prescription may be made in primary care.
- For people with an established diagnosis of Alzheimer’s disease who are already taking an AChE inhibitor, primary care prescribers may start treatment with memantine without taking advice from a specialist clinician.
- Do not stop AChE inhibitors in people with Alzheimer’s disease because of disease severity alone.
- If prescribing an AChE inhibitor (donepezil, galantamine or rivastigmine), treatment should normally be started with the drug with the lowest acquisition cost (taking into account required daily dose and the price per dose once shared care has started). However, an alternative AChE inhibitor could be prescribed if it is considered appropriate when taking into account adverse event profile, expectations about adherence, medical comorbidity, possibility of drug interactions and dosing profiles (see below).
NHS Somerset Formulary for Interactions, see Appendix 1
- Concomitant use of anticholinergics with cholinesterase inhibitors reduces the effectiveness of antidementia drugs, concomitant use increases the risk of adverse effects of anticholinergics and may also increase the rate of functional and cognitive decline.
- Memantine monotherapy is recommended as an option for managing Alzheimer’s disease for people with:
• moderate Alzheimer’s disease who are intolerant of or have a contraindication to AChE inhibitors or
• severe Alzheimer’s disease. - For people with an established diagnosis of Alzheimer’s disease who are already taking an AChE inhibitor:
• consider memantine in addition to an AChE inhibitor if they have moderate disease
• offer memantine in addition to an AChE inhibitor if they have severe disease. - When using assessment scales to determine the severity of Alzheimer’s disease, healthcare professionals should take into account any physical, sensory or learning disabilities, or communication difficulties that could affect the results and make any adjustments they consider appropriate. Healthcare professionals should also be mindful of the need to secure equality of access to treatment for patients from different ethnic groups, in particular those from different cultural backgrounds.
- When assessing the severity of Alzheimer’s disease and the need for treatment, healthcare professionals should not rely solely on cognition scores in circumstances in which it would be inappropriate to do so.
These include:
• if the cognition score is not, or is not by itself, a clinically appropriate tool for assessing the severity of that patient’s dementia because of the patient’s learning difficulties or other disabilities (for example, sensory impairments), linguistic or other communication difficulties or level of education or
• if it is not possible to apply the tool in a language in which the patient is sufficiently fluent for it to be appropriate for assessing the severity of dementia
or
• if there are other similar reasons why using a cognition score, or the score alone, would be inappropriate for assessing the severity of dementia.
In such cases healthcare professionals should determine the need for initiation or continuation of treatment by using another appropriate method of assessment. - Do not offer the following specifically to slow the progress of Alzheimer’s disease, except as part of a randomised controlled trial:
• diabetes medicines
• hypertension medicines
• statins
• non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin.
Pharmacological management of non-Alzheimer’s dementia
- Offer donepezil or rivastigmine to people with mild to moderate dementia with Lewy bodies (off label).
- Only consider galantamine for people with mild to moderate dementia with Lewy bodies if donepezil and rivastigmine are not tolerated (off label).
- Consider donepezil or rivastigmine for people with severe dementia with Lewy bodies (off label).
- Consider memantine for people with dementia with Lewy bodies if AChE inhibitors are not tolerated or are contraindicated (off label).
- Only consider AChE inhibitors or memantine for people with vascular dementia if they have suspected comorbid Alzheimer’s disease, Parkinson’s disease dementia or dementia with Lewy bodies (off label).
- Do not offer AChE inhibitors or memantine to people with frontotemporal dementia. Note that logopenic aphasia, which has previously been included in some diagnostic guidelines for frontotemporal dementia, has now been shown to most commonly be caused by Alzheimer’s disease.
- Do not offer AChE inhibitors or memantine to people with cognitive impairment caused by multiple sclerosis.
- For guidance on pharmacological management of Parkinson’s disease dementia, see Parkinson’s disease dementia in the NICE guideline on Parkinson’s disease.
Medicines that may cause cognitive impairment
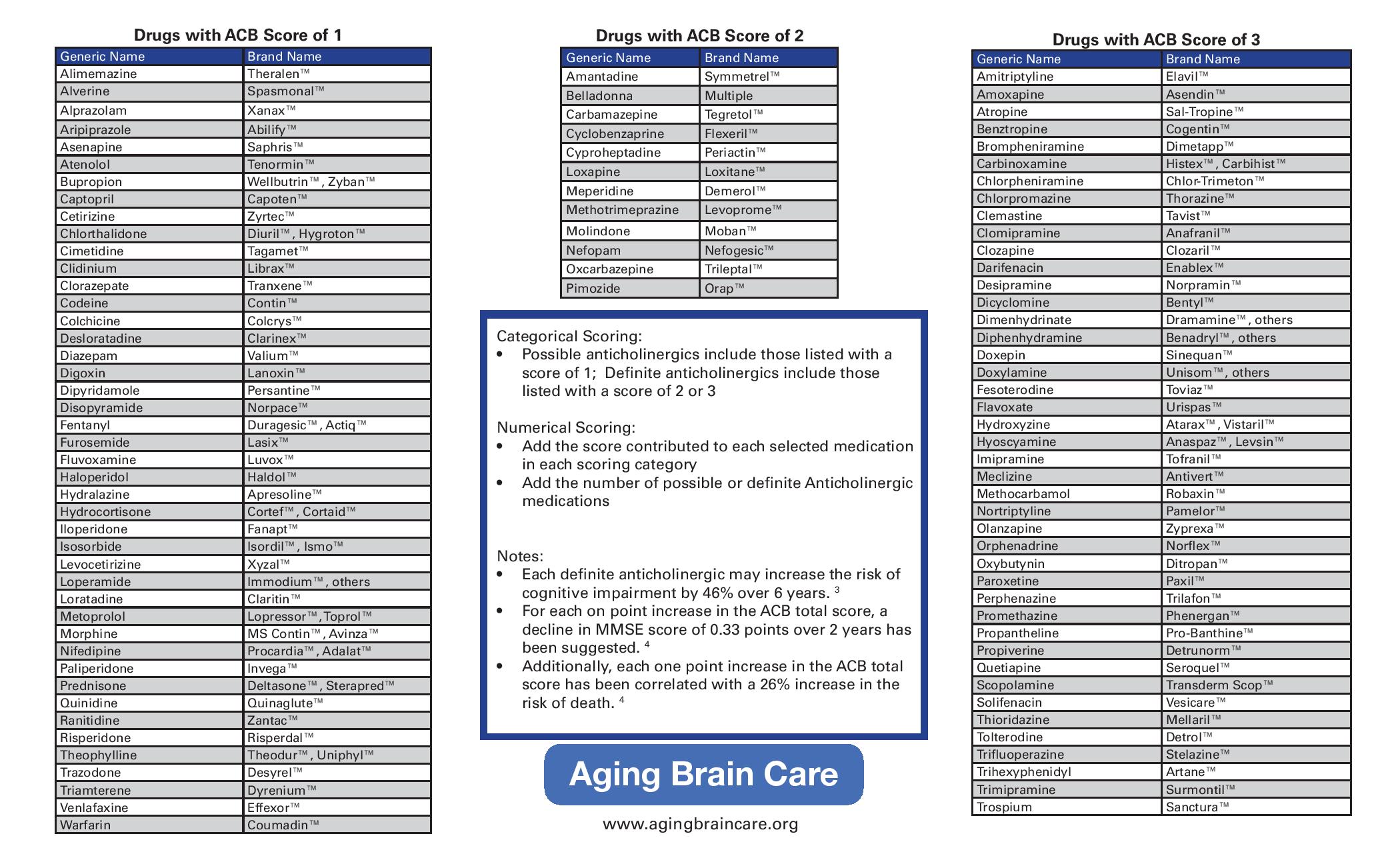
- Be aware that some commonly prescribed medicines are associated with increased anticholinergic burden, and therefore cognitive impairment.
- Consider minimising the use of medicines associated with increased anticholinergic burden, and if possible look for alternatives:
• when assessing whether to refer a person with suspected dementia for diagnosis
• during medication reviews with people living with dementia. - Be aware that there are validated tools for assessing anticholinergic burden (for example, the Anticholinergic Cognitive Burden Scale), but there is insufficient evidence to recommend one over the others.
Agitation, aggression, distress and psychosis
- Before starting non-pharmacological or pharmacological treatment for distress in people living with dementia, conduct a structured assessment to:
• explore possible reasons for their distress and
• check for and address clinical or environmental causes (for example pain, delirium or inappropriate care). - As initial and ongoing management, offer psychosocial and environmental interventions to reduce distress in people living with dementia.
- Only offer antipsychotics for people living with dementia who are either:
• at risk of harming themselves or others or
• experiencing agitation, hallucinations or delusions that are causing them severe distress. - Follow the MHRA advice for health and social care professionals on prescribing antipsychotics to people living with dementia.
See Antipsychotics: initiative to reduce prescribing to older people with dementia (MHRA 2014)
- Be aware that for people with dementia with Lewy bodies or Parkinson’s disease dementia, antipsychotics can worsen the motor features of the condition, and in some cases cause severe antipsychotic sensitivity reactions. Be aware that interventions may need to be modified for people living with dementia.
- Before starting antipsychotics, discuss the benefits and harms with the person and their family members or carers (as appropriate). Consider using a decision aid to support this discussion.
See NHS Somerset Formulary Psychoses and schizophrenia.
- When using antipsychotics:
• use the lowest effective dose and use them for the shortest possible time
• reassess the person at least every 6 weeks, to check whether they still need medication. - Stop treatment with antipsychotics:
• if the person is not getting a clear ongoing benefit from taking them and
• after discussion with the person taking them and their family members or carers (as appropriate). - Ensure that people living with dementia can continue to access psychosocial and environmental interventions for distress while they are taking antipsychotics and after they have stopped taking them.
- For people living with dementia who experience agitation or aggression, offer personalised activities to promote engagement, pleasure and interest.
- Do not offer valproate to manage agitation or aggression in people living with dementia, unless it is indicated for another condition.
Depression and anxiety
- For people living with mild to moderate dementia who have mild to moderate depression and/or anxiety, consider psychological treatments.
- Do not routinely offer antidepressants to manage mild to moderate depression in people living with mild to moderate dementia, unless they are indicated for a pre-existing severe mental health problem.
See NHS Somerset Formulary for antidepressants see Depression
See NHS Somerset Formulary Anxiety
Sleep problems
- Do not offer melatonin to manage insomnia in people living with Alzheimer’s disease.
- For people living with dementia who have sleep problems, consider a personalised multicomponent sleep management approach that includes sleep hygiene education, exposure to daylight, exercise and personalised
activities.
See NHS Somerset Formulary Sleep disorders
Parkinson’s disease
- For guidance on managing Parkinson’s disease symptoms in people with Parkinson’s disease dementia or dementia with Lewy bodies, see the NICE guideline on Parkinson’s disease. Be aware that interventions may
need to be modified for people living with dementia.
For Parkinson’s disease see NHS Somerset Formulary Movement disorders
Assessing and managing other long-term conditions in people living with dementia
- Ensure that people living with dementia have equivalent access to diagnosis, treatment and care services for comorbidities to people who do not have dementia. For more guidance on assessing and managing multimorbidity, see the NICE guidelines on multimorbidity and older people with social care needs and multiple long-term conditions.
- For more guidance on providing support for older adults with learning disabilities, see the NICE guideline on care and support of people growing older with learning disabilities.
Pain
- Consider using a structured observational pain assessment tool:
• alongside self-reported pain and standard clinical assessment for people living with moderate to severe dementia
• alongside standard clinical assessment for people living with dementia who are unable to self-report pain. - For people living with dementia who are in pain, consider using a stepwise treatment protocol that balances pain management and potential adverse events.
- Repeat pain assessments for people living with dementia:
• who seem to be in pain
• who show signs of behavioural changes that may be caused by pain
• after any pain management intervention.
See NHS Somerset Formulary Pain
Falls
- For guidance on managing the risk of falling for people living with dementia (in community and inpatient settings), see the NICE guideline on falls in older people. When using this guideline:
• take account of the additional support people living with dementia may need to participate effectively
• be aware that multifactorial falls interventions may not be suitable for a person living with severe dementia.
Diabetes
- For guidance on setting HbA1c targets for people living with severe dementia who have type 2 diabetes, see the NICE guideline on type 2 diabetes in adults.
- Consider relaxing the target HbA1c level on a case-by-case basis and in discussion with adults with type 2 diabetes, with particular consideration for people who are older or frailer, if: they are unlikely to achieve longer-term risk-reduction benefits, for example, people with a reduced life expectancy, tight blood glucose control would put them at high risk if they developed hypoglycaemia, for example, if they are at risk of falling, they have impaired awareness of hypoglycaemia, or they drive or operate machinery as part of their job, intensive management would not be appropriate, for example if they have significant comorbidities.
See NHS Somerset Formulary Diabetes
Incontinence
- For guidance on pharmacological treatment of overactive bladder, see the NICE technology appraisal on mirabegron for treating symptoms of overactive bladder.
See NHS Somerset Formulary Urinary frequency, enuresis, and incontinence.
- For guidance on treating faecal incontinence, see the NICE guideline on faecal incontinence.
- Healthcare professionals should take a proactive approach to bowel management for specific groups of people;
See NHS Somerset Formulary Constipation and bowel cleansing.
- If baseline assessment and initial management have failed to resolve faecal incontinence, people with confirmed severe cognitive impairment should be referred for a behavioural and functional analysis to determine if there is any behavioural reason for faecal incontinence. Following analysis, people should be offered cause-specific interventions founded on structured goal planning that aim to resolve as well as manage behavioural aspects that may be contributing to faecal incontinence. In cases of severe cognitive impairment, further specialist management of faecal incontinence may be inappropriate.
Sensory impairment
- For guidance on hearing assessments for people with suspected or diagnosed dementia, see adults with suspected dementia in the NICE guideline on hearing loss.
- Encourage people living with dementia to have eye tests every 2 years. Consider referring people who cannot organise appointments themselves.
Palliative care
- From diagnosis, offer people living with dementia flexible, needs-based palliative care that takes into account how unpredictable dementia progression can be.
- For people living with dementia who are approaching the end of life, use an anticipatory healthcare planning process. Involve the person and their family members or carers (as appropriate) as far as possible, and use the principles of best-interest decision-making if the person does not have capacity to make decisions about their care.
- For standards and measures on palliative care, see the NICE quality standard on end of life care for adults.
- For guidance on care for people in the last days of life, see the NICE guideline on care of dying adults.
- For guidance on best interests decision-making, see the NICE guideline on decision-making and mental capacity.
- Encourage and support people living with dementia to eat and drink, taking into account their nutritional needs.
See NHS Somerset Formulary Blood and Nutrition
for The Caroline Walker Trust Eating well: supporting older people and older people with dementia practical guide. (2014)
- Consider involving a speech and language therapist if there are concerns about a person’s safety when eating and drinking.
- Do not routinely use enteral feeding in people living with severe dementia, unless indicated for a potentially reversible comorbidity.
NICE patient decision aid on enteral (tube) feeding for people living with severe dementia.
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Anticholinesterases, centrally acting | Donepezil | 5mg tablet: £1.18 | NHS Somerset classify as an Amber drug for mild to moderate dementia in Alzhelimer's disease as per traffic light guidance and shared care protocol. Adult: Initially 5 mg once daily for one month, then increased if necessary up to 10 mg daily, doses to be given at bedtime. |
| 10mg tablet: £1.36 | |||
| 5mg orodispersible tablet sugar free: £31.68 | |||
| 10mg orodispersible tablet sugar free: £87.31 | |||
| 1mg/ml oral solution sugar free: £97.00 (150ml) | |||
| Galantamine as Galzemic XL® | 8mg modified-release capsule: £19.03 | NHS Somerset classify as an Amber drug for mild to moderate dementia in Alzhelimer's disease as per traffic light guidance and shared care protocol. Modified-release capsule. Adult: Initially 8 mg once daily for 4 weeks, increased to 16 mg once daily for at least 4 weeks; maintenance 16–24 mg daily. Immediate release oral solution. Adult: Initially 4 mg twice daily for 4 weeks, increased to 8 mg twice daily for at least 4 weeks; maintenance 8–12 mg twice daily. |
|
| 16mg modified-release capsule: £23.82 | |||
| 24mg modified-release capsule: £29.30 | |||
| 20mg/5ml oral solution sugar free: £90.00 (100ml) | |||
| Gazylan XL® | 8mg modified-release capsule: £19.04 | ||
| 16mg modified-release capsule: £23.83 | |||
| 24mg modified-release capsule: £29.31 | |||
| Luventa XL® | 8mg modified-release capsule: £25.42 | ||
| 16mg modified-release capsule: £31.80 | |||
| 24mg modified-release capsule: £39.10 | |||
| Gatalin XL® | 8mg modified-release capsule: £25.94 | ||
| 16mg modified-release capsule: £32.45 | |||
| 24mg modified-release capsule: £39.90 | |||
| Rivastigmine | 1.5mg capsule: £3.20 | NHS Somerset classify as an Amber drug for mild to moderate dementia in Alzhelimer's disease and Parkinson's disease as per traffic light guidance and shared care protocol. Adult: Initially 1.5 mg twice daily, increased in steps of 1.5 mg twice daily, dose to be increased at intervals of at least 2 weeks according to response and tolerance; usual dose 3–6 mg twice daily (max. per dose 6 mg twice daily), if treatment interrupted for more than several days, retitrate from 1.5 mg twice daily. By transdermal application using patches. Adult: Apply 4.6 mg/24 hours daily for at least 4 weeks, increased if tolerated to 9.5 mg/24 hours daily for a further 6 months, then increased if necessary to 13.3 mg/24 hours daily, increase to 13.3 mg/24 hours patch if well tolerated and cognitive deterioration or functional decline demonstrated; use caution in patients with body-weight less than 50 kg, if treatment interrupted for more than 3 days, retitrate from 4.6 mg/24 hours patch. |
|
| 3mg capsule: £3.84 | |||
| 4.5mg capsule: £23.53 | |||
| 6mg capsule: £27.88 | |||
| 2mg/ml oral solution sugar free: £96.82 (120ml) | |||
| as Alzest® | 4.6mg/24hr patch: £35.10 (30) | ||
| 9.5mg/24hr patch: £19.97 (30) | |||
| 13.3mg/24hours transdermal patch: £54.58 (30) | |||
| Dopaminergic drugs, NMDA receptor antagonist | Memantine | 10mg tablet: £1.17 | NHS Somerset classify as an Amber drug for moderate to severe dementia in Alzhelimer's disease as per traffic light guidance and shared care protocol. Adult: Initially 5 mg once daily, then increased in steps of 5 mg every week; usual maintenance 20 mg daily; maximum 20 mg per day. |
| 20mg tablet: £1.46 | |||
| 10mg/ml oral solution sugar free: £6.28 (50ml) |