4.4.2 – Parkinson’s disease
Related guidance:
Parkinson’s disease in adults NICE guideline (NG71 July 2017)
- Do not offer anticholinergics to people with Parkinson’s disease who have developed dyskinesia and/or motor fluctuations.
Orthostatic hypotension due to autonomic dysfunction: midodrine Evidence summary (ESNM61
Restless legs syndrome (NICE CKS July 2022)
- Restless legs syndrome (RLS) is a neurological disorder characterized by an irresistible urge to move the limbs (usually the legs) accompanied by uncomfortable sensations. Symptoms are typically worse in the evenings, and are often associated with sleep disturbance.
- The underlying pathophysiology of RLS is not fully understood, although it is likely that there is dysfunction of the dopaminergic system.
- RLS is frequently idiopathic but may be secondary to an underlying condition (most commonly pregnancy, iron deficiency, or stage 5 chronic kidney disease), or the use of certain drugs (for example, some antidepressants, some antipsychotics, and lithium).
- In idiopathic RLS the severity and frequency of symptoms typically increase over time.
- For a diagnosis of RLS to be made, the following criteria must all be present:
- An urge to move the legs, usually accompanied by, or felt to be caused by, unpleasant sensations.
- Symptoms begin or worsen during periods of rest or inactivity such as lying down or sitting.
- Symptoms are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues.
- Symptoms during rest or inactivity only occur, or are worse, in the evening or night.
- The above features are not primarily caused by another medical or behavioural condition (for example, leg cramps or habitual foot tapping).
- Differential diagnoses of RLS include: nocturnal leg cramps, peripheral neuropathy, akathisia, and intermittent claudication.
- There are no specific investigations to confirm the diagnosis of idiopathic RLS. The following investigations may help identify an underlying cause:
- Serum ferritin (to identify iron deficiency).
- Other investigations (such as renal function, full blood count, thyroid function, blood glucose, vitamin B12) as guided by the history and examination.
- Any underlying cause or exacerbating factors of RLS should be addressed, as symptoms may resolve if the underlying condition is treated.
- Self-help advice that may ease symptoms of RLS include measures to:
- Prevent an attack, such as good sleep hygiene, reducing caffeine and alcohol consumption, stopping smoking, and taking moderate regular exercise.
- Relieve an attack, such as walking and stretching, application of heat with heat pads or a hot bath, relaxation exercises, mental distraction at times of rest, and massaging affected limbs.
- For people with mild symptoms, explanation, reassurance, and self-help measures may be sufficient.
- For people with moderate to severe symptoms, drug treatment is an option with either a non-ergot dopamine agonist (pramipexole, ropinirole, or rotigotine), or an alpha-2-delta ligand (pregabalin or gabapentin — both off-label indications).
- A weak opioid (such as codeine) is an alternative for people with painful symptoms.
- A short or intermittent course of a hypnotic drug (such as a Z-drug) may be considered for people with significant sleep disturbance due to RLS.
- Referral to a neurologist or sleep specialist may be required if there is doubt about the diagnosis, or if treatment is unsuccessful.
For opioids see NHS Somerset formulary Pain.
For hypnotics see NHS Somerset formulary Sleep disorders.
For pregabalin or gabapentin see NHS Somerset formulary Epilepsy and other seizure disorders.
See National medicines optimisation opportunities 2023/24 (NHSE 2023)
Parkinson’s disease medicines formulations for adults with swallowing difficulties (SPS June 2022)
Emergency management of patients with Parkinson’s (Parkinson’s UK November 2013)
See Somerset NHS Foundation Trust Parkinson’s disease service.
See Somerset NHS Foundation Trust Dietetics service.
See NHS Somerset formulary feed thickeners.
See NHS Somerset specials guidance.
- Communication with people with Parkinson’s disease should aim towards empowering people to participate in judgements and choices about their own care with an aim to achieve a balance between providing honest, realistic information about the condition and promoting a feeling of optimism. Because people with Parkinson’s disease may develop impaired cognitive ability, communication problems and/or depression, provide them with and record both oral and written communication throughout the course of the disease, which should be individually tailored and reinforced as necessary with consistent communication from professionals involved.
- Advise family members and carers about their right to carer assessment and assessment for respite care and other support. See the NICE guideline on supporting adult carers for recommendations on identifying, assessing and meeting the caring, physical and mental health needs of families and carers.
- People with Parkinson’s disease should have a comprehensive care plan agreed between the person, their family members, carers and specialist healthcare providers, offered an accessible point of contact with specialist services and advised to inform the Driver and Vehicle Licensing Agency (DVLA) and their car insurer of their condition when Parkinson’s disease is diagnosed.
- Suspect Parkinson’s disease in people presenting with tremor, stiffness, slowness, balance problems and/or gait disorders. If Parkinson’s disease is suspected, refer people quickly and untreated to a specialist with expertise in the differential diagnosis of this condition.
- The diagnosis of Parkinson’s disease must be regularly reviewed and reconsidered if atypical clinical features develop.
- Do not use vitamin E, co-enzyme Q10, dopamine agonists, or MAO-B inhibitors as a neuroprotective therapy for people with Parkinson’s disease.
Dopaminergic treatment
- Offer levodopa to people in the early stages of Parkinson’s disease whose motor symptoms impact on their quality of life and consider a choice of dopamine agonists, levodopa or monoamine oxidase B (MAO-B) inhibitors for people in the early stages of Parkinson’s disease whose motor symptoms do not impact on their quality of life. Ergot-derived dopamine agonists should not be offered as first-line treatment for Parkinson’s disease.
- Parkinson’s medication should be prescribed at specific times and administered on time. NPSA alert 2010 highlighted people with Parkinson’s disease who do not receive their medicines on time may recover slowly after illness or lose function, such as ability to walk.
- Abrupt withdrawal or failure due to poor absorption (for example, gastroenteritis, abdominal surgery) should be avoided due to the potential for acute loss of movement or neuroleptic malignant syndrome and should be adjusted only after discussion with a specialist in the management of Parkinson’s disease.
- If a person with Parkinson’s disease has developed dyskinesia and/or motor fluctuations, including medicines ‘wearing off’, seek advice from specialist before modifying therapy. Dopamine agonists, MAO-B inhibitors or catechol-O-methyl transferase (COMT) inhibitors may be offered as an adjunct to levodopa for people with Parkinson’s disease who have developed dyskinesia or motor fluctuations despite optimal levodopa therapy. If dyskinesia is not adequately managed by modifying existing therapy, amantadine may be considered. NHS Somerset classify amantadine as an Amber drug as per Traffic light guidance.
- Do not offer anticholinergics to people with Parkinson’s disease who have developed dyskinesia and/or motor fluctuations.
- Do not use acute levodopa and apomorphine challenge tests in the differential diagnosis of parkinsonian syndromes.
- Offer people with advanced Parkinson’s disease best medical therapy, which may include intermittent apomorphine injection and/or continuous subcutaneous apomorphine infusion.
NHS Somerset classify Apomorphine a red drug as per Traffic light guidance.
- Do not offer deep brain stimulation to people with Parkinson’s disease whose symptoms are adequately controlled by best medical therapy.
- Levodopa–carbidopa intestinal gel is only available via tertiary specialist centres.
Swallowing difficulties
- Swallowing difficulties may develop and can be unpredictable and variable. Advice should be sought from the Parkinson’s Disease specialist team, particularly for changes to complex medication regimens with a rescue plan in place to prioritise dopaminergic and other essential medication during this period. Some formulations can be crushed if needed, see SPS guidance. Dispersible Co-beneldopa is a useful option during swallowing difficulties and can be used with thickener as per Speech and language therapist (SALT) advice, it has a faster onset and shorter duration of action. Transdermal dopamine agonist may be considered if oral route is no longer reliable to avoid acute loss of movement or neuroleptic malignant syndrome.
Impulse control disorders
- Impulse control disorders (e.g. compulsive gambling, hypersexuality, binge eating and obsessive shopping) can develop in a person with Parkinson’s disease who is on any dopaminergic therapy at any stage in the disease course. Dopamine agonist therapy, history of previous impulsive behaviours and a history of alcohol consumption and/or smoking increases this risk. Refer to specialist as therpay may need to be modified. Specialist cognitive behavioural therapy targeted at impulse control disorders may be offered if modifying dopaminergic therapy is not effective.
Psychotic symptoms
- Patients may experience psychotic symptoms (hallucinations and delusions) with all Parkinson’s disease treatments (higher risk with dopamine agonists). Refer to specialist if person is experiencing hallucinations (particularly visual) or delusions as medication may need to be modified. Do not treat hallucinations and delusions if they are well tolerated by the person with Parkinson’s disease and their family members and carers. Quetiapine may be considered by specialist to treat hallucinations and delusions in people with Parkinson’s disease who have no cognitive impairment. If standard treatment is not effective, clozapine may be offered to treat hallucinations and delusions in people with Parkinson’s disease. Be aware that registration with a patient monitoring service is needed and that lower doses of quetiapine and clozapine are needed for people with Parkinson’s disease than in other indications.
- Olanzapine should not be used to treat hallucinations and delusions in people with Parkinson’s disease. Antipsychotic medicines (such as phenothiazines and butyrophenones) can worsen the motor features of Parkinson’s disease.
See NHS Somerset Formulary Psychoses and schizophrenia.
- For hallucinations and delusions in people with dementia, conduct a structured assessment to explore possible reasons for their distress and check for and address clinical or environmental causes (e.g. pain, delirium or inappropriate care) before starting non-pharmacological or pharmacological treatment. As initial and ongoing management, offer psychosocial and environmental interventions to reduce distress in people living with dementia. Only offer antipsychotics for people living with dementia who are either at risk of harming themselves or others or experiencing agitation, hallucinations or delusions that are causing them severe distress.
See NHS Somerset Formulary Dementia.
Dementia
- A cholinesterase inhibitor such as rivastigmine or donepezil, galantamine and rivastigmine patches (off-label) may be offered by a specialist for people with mild or moderate Parkinson’s disease dementia. Consider a cholinesterase inhibitor for people with severe Parkinson’s disease dementia (off-label) and memantine (off-label) only if cholinesterase inhibitors are not tolerated or are contraindicated. For guidance on assessing and managing dementia, and supporting people living with dementia, see the NICE guideline on dementia. See NHS Somerset Shared Care Protocol for Dementia.
See NHS Somerset Formulary Dementia.
Insomnia
- Melatonin is a useful option for Parkinson’s disease related insomnia on the recommendation of secondary care as per Traffic light guidance as of benefit to elderly patients at risk of falling, or to people who drive and are susceptible to next-day drowsiness of z-drugs and benzodiazepines, in addition avoidance of associated dependance and withdrawal. See NICE’s guideline on medicines associated with dependence or withdrawal symptoms.
NHS Somerset formulary Sleep disorders.
Nocturnal akinesia and orthostatic hypotension
- Refer to specialist for the management of nocturnal akinesia and orthostatic hypotension as a review of existing medicines including antihypertensives (including diuretics), dopaminergics, anticholinergics and antidepressants may be required. Non-pharmacological management options are recommended first-line for orthostatic hypotension include compression stockings, blood pressure monitoring and increased water and salt ingestion. Midodrine may be considered second line, taking into account the contraindications and monitoring requirements (including monitoring for supine hypertension). If midodrine is contraindicated, not tolerated or not effective, consider fludrocortisone (off-label), taking into account its safety profile, in particular its cardiac risk and potential interactions with other medicines.
NHS Somerset classify midodrine as an Amber drug as per Traffic light guidance.
Excessive day time sleepiness
- Dopamine agonists may cause excessive day time sleepiness. Advise people not to drive and to inform the DVLA of their symptoms and consider any occupation hazards. Refer to specialist for medication adjustment to reduce occurrence.
- Consider modafinil to treat excessive daytime sleepiness in people with Parkinson’s disease, only if a detailed sleep history has excluded reversible pharmacological and physical causes.
NHS Somerset classify modafinil as an Amber drug as per Traffic light guidance.
Women who are pregnant or who are planning a pregnancy should not take modafinil because it may increase the risk of congenital defects. See MHRA alert Modafinil: increased risk of congenital malformations if used during pregnancy November 2020.
Restless legs syndrome and rapid eye movement sleep behaviour disorder
- Take care to identify and manage restless legs syndrome (see above) and rapid eye movement sleep behaviour disorder in people with Parkinson’s disease and sleep disturbance and refer to specialist.
- Consider clonazepam or melatonin to treat rapid eye movement sleep behaviour disorder if a medicines review has addressed possible pharmacological causes (off-label).
For melatonin see NHS Somerset formulary Sleep disorders.
For clonazepam see NHS Somerset formulary Epilepsy and other seizure disorders.
Depression
- For guidance on identifying, treating and managing depression in people with Parkinson’s disease, see NICE’s guideline on depression in adults with a chronic physical health problem.
For antidepressants see NHS Somerset formulary Depression.
- Fluoxetine or fluvoxamine would not be considered a first line option in people with Parkinson’s disease when starting an antidepressant as may impact on future Parkinson’s disease medication options e.g. increased risk of serotonin syndrome if taken in combination with MAOIs e.g. Rasagiline and Selegiline and atypical antipsychotics e.g. quetiapine and clozapine.
Drooling
- Only consider pharmacological management for drooling of saliva in people with Parkinson’s disease if non-pharmacological management e.g. speech and language therapy is not available or has not been effective. Consider glycopyrronium bromide to manage drooling of saliva in people with Parkinson’s disease (off-label). Only consider anticholinergic medicines e.g. hyoscine hydrobromide other than glycopyrronium bromide to manage drooling of saliva in people with Parkinson’s disease if their risk of cognitive adverse effects is thought to be minimal. Topical preparations can be considered to reduce the risk of adverse events e.g. Hyoscine patch (off-label). Atropine is non formulary. If these treatment options are not effective, not tolerated or contraindicated e.g. in people with cognitive impairment, hallucinations or delusions, or a history of adverse effects following anticholinergic treatment consider referral to a specialist service for botulinum toxin A.
NHS Somerset classify botulinum toxin A as a Red drug, as per Traffic light guidance.
Constipation
- Constipation often affects those with Parkinson’s disease due to improper functioning of the autonomic nervous system, responsible for regulating smooth muscle activity which can result in the intestinal tract operating slowly. The normal length of time between bowel movements ranges widely from person to person. Some people have bowel movements three times a day; others only one to two times a week. Going longer than three days without a bowel movement causes the stool to harden and become more difficult to pass.The most common causes include:
- not eating enough fibre, which is found in fruits, vegetables and cereals
- not drinking enough fluids
- not moving enough and spending long periods sitting or lying down
- being less active and not exercising
- often ignoring the urge to go to the toilet
- changing your diet or daily routine
- a side effect of medicine e.g. anticholinergics
- stress, anxiety or depression
- If you are caring for someone with dementia, constipation may be easily missed. Look out for any behaviour changes, as it might mean they are in pain or discomfort.
- See The Association of UK Dieticians Feeling bunged up? Don’t let poo be a taboo
For laxatives see NHS Somerset formulary Constipation and bowl cleansing.
Nausea and vomiting
- Domperidone should be considered first line if an anti-emetic is needed.
For anti-emetics see Somerset NHS formulary Nausea and labyrinth disorders.
- Metoclopramide should be avoided due to extrapyramidal effects and cyclizine used with caution due to potential extrapyramidal and anti-cholinergic effects.
People who continue treatment should be reviewed regularly by specialist in the management of Parkinson’s disease.
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Dopaminergic drugs | |||
| Levodopa with decarboxylase inhibitors | Co-beneldopa (Levodopa/Benserazide) | ||
| 12.5/50mg capsule: £4.96 (100) | NHS Somerset classify as an Amber drug for Parkinson's disease. | ||
| 25/100mg capsule: £6.91 (100) |
|||
| 50/200mg capsule: £11.78 (100) | |||
| 25/100mg modified release capsule: £12.77 (100) | Modified release is not levodopa equivalent. See Parkinson’s UK Calculate Adjusted Levodopa Equivalent Daily Dose. |
||
| 12.5/50mg dispersible tablet: £5.90 (100) | Dispersible has a faster onset and shorter duration of action. | ||
| 50/100mg dispersible tablet: £10.45 (100) | |||
| Co-careldopa (Levodopa/Carbidopa) | NHS Somerset classify as an Amber drug for Parkinson's disease. | ||
| 12.5/50mg tablet: £3.37 (90) | |||
| 10/100mg tablet: £13.98 (100) | |||
| 25/100mg tablet: £5.87 (100) | |||
| 25mg/250mg tablet: £35.23 (100) | |||
| 25/100mg modified-release tablet:£11.60 (60) | Modified release is not levodopa equivalent. See Parkinson’s UK Calculate Adjusted Levodopa Equivalent Daily Dose. |
||
| 50/200mg modified-release tablet: £11.60 (60) | |||
| Catechol-o-methyltransferase inhibitors | Entacapone | 200mg tablet: £3.07 (30) | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 2g daily. |
| Opicapone | 50mg capsule: £93.90 (30) | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 50mg daily. |
|
| NHS Somerset classify Tolcapone as a Red drug (specialist prescribing only) as per Traffic light guidance. | |||
| Levodopa with catechol-o-methyltransferase inhibitor | Levodopa/ Carbidopa/ Entacapone as Sastravi or Stanek® | 50mg/12.5mg/200mg tablet: £10.35 (30) £34.66 (100) | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 10 tablets per day. |
| 75mg/18.75mg/200mg tablet: £10.35 (30) £34.66 (100) | |||
| 100mg/25mg/200mg tablet: £10.35 (30) £34.66 (100) | |||
| 125mg/31.25mg/200mg tablet: £10.35 (30) £34.66 (100) | |||
| 150mg/37.5mg/200mg tablet: £10.35 (30) £34.66 (100) | |||
| 175mg/43.75mg/200mg tablet: £10.35 (30) £34.66 (100) | Maximum 8 tablets per day. | ||
| 200mg/50mg/200mg tablet: £10.35 (30) £34.66 (100) | Maximum 7 tablets per day. | ||
| Dopamine receptor agonists | Amantadine | 100mg capsule: £13.67 (56) | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 400mg daily. |
| 10 mg per 1 ml oral solution sugar free: £140 (150ml) | |||
| Ropinirole | 250 mcg tablet: £3.99 (12) | For Parkinson's disease, used alone or as an adjunct to co-beneldopa or co-careldopa and moderate to severe restless legs syndrome. Maximum 24mg daily. |
|
| 500 mcg tablet: £7.08 | |||
| 1 mg tablet: £49.84 (84) | |||
| 2 mg tablet: £19.14 | |||
| 5 mg tablet: £214.25 (84) | |||
| 2 mg modified-release tablet: £12.54 | |||
| 4mg modified-release tablet: £25.09 | |||
| 6mg modified-release tablet: £15.32 | |||
| 8mg modified-release tablet: £42.11 | |||
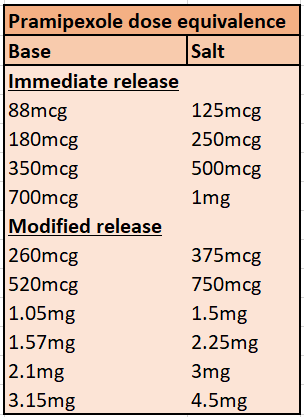
| Pramipexole | 88 mcg tablet: £2.10 (30) | For Parkinson's disease, used alone or as an adjunct to co-beneldopa or co-careldopa and moderate to severe restless legs syndrome. Maximum 3.3 mg base daily. See table below for equivalence and dose conversion. |
|
| 180 mcg tablet : £1.49 (30) | |||
| 350 mcg tablet: £15.03 (30) | |||
| 700 mcg tablet: £1.80 (30) | |||
| 260 mcg modified-release tablet: £30.81 (30) | Maximum 3.15 mg base daily. See table below for equivalence and dose conversion. |
||
| 520 mcg modified-release tablet: £60.11 (30) | |||
| 1.05 mg modified-release tablet : £119.76 (30) | |||
| 1.57 mg modified-release tablet: £191.90 (30) | |||
| 2.1 mg modified-release tablet: £246.46 (30) | |||
| 2.62 mg modified-release tablet: £312.10 (30) | |||
| 3.15 mg modified-release tablet: £369.71 (30) | |||
| NHS Somerset classify Cabergoline as a Red drug (specialist prescribing only) as per Traffic light guidance. | |||
| Rotigotine | 2 mg per 24 hour transdermal patch: £81.10 | NHS Somerset classify as an Amber drug for Parkinson's disease patients with unreliable swallow/absorption of oral medication and for moderate to severe restless legs syndrome. Maximum 16mg daily. Apply the patch to a different place on your skin every day – use the body map as a guide to locate appropriate areas to place the patch. |
|
| 4 mg per 24 hour transdermal patch: £123.60 | |||
| 6 mg per 24 hour transdermal patch: £149.93 | |||
| 8 mg per 24 hour transdermal patch: £149.93 | |||
| NHS Somerset classify Apomorphine as a Red drug (specialist prescribing only) as per Traffic light guidance. | |||
| Monoamine oxidase B inhibitors | Rasagiline | 1mg tablet: £3.81 | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 1mg daily. |
| Selegiline | 5mg tablet: £16.52 (100) | NHS Somerset classify as an Amber drug for Parkinson's disease as per Traffic light guidance. Maximum 10mg daily. |
|
| Safinamide | 50mg tablet: £69.00 (30) | NHS Somerset classify as an Amber drug for Parkinson's disease as an option if first line Rasagiline and Selegiline are not effective or contraindicated as per Traffic light guidance. Maximum 100mg daily. |
|
| 100mg tablet: £69.00 (30) | |||
| Sympathomimetic- Vasoconstrictor | Midodrine | 2.5mg tablet: £28.73 (100) | NHS Somerset classify as an Amber drug for the treatment of orthostatic hypotension per Traffic light guidance. The usual initial dosage is 2.5 mg, 2-3 times daily (every 3-4 hours). The dose should be increased at weekly intervals in small increments until an optimal response is obtained (max 30 mg/day) and taken during daytime when the person performs daily activities in upright position. The last dose should be taken at least four hours before bedtime to reduce the risk of supine hypertension. Blood pressure in supine and sitting position should be regularly monitored at the beginning of the treatment (at least twice a week). Treatment should be stopped if supine hypertension is significantly excessive. |
| 5mg tablet: £41.66 (100) | |||
| 10mg tablet: £83.32 (100) | |||
| Corticosteroid | Fludrocortisone | 100mcg tablet: £10.08 (30) | NHS Somerset classify as an Amber drug for the treatment of orthostatic hypotension (off-label) if midodrine is contraindicated, not tolerated or not effective, taking into account its safety profile, in particular its cardiac risk and potential interactions with other medicines. Adult: 100-400mcg daily. |
| Centrally acting sympathomimetic | Modafinil | 100mg tablet: £2.92 (30) | NHS Somerset classify modafinil as an Amber drug for the treatment of excessive sleepiness in people with Parkinson's disease. Adult: 200-400mg morning or in divided doses (morning and noon). Elderly: initially 100 mg daily. Women who are pregnant or who are planning a pregnancy should not take modafinil because it may increase the risk of congenital defects. |
| Antimuscarinic Antimuscarininics can cause cognitive impairment, urinary retention and constipation which should be monitored closely. | Glycopyrronium bromide as Assicco® | 1mg tablet: £79.00 (30) | For the treatment of severe drooling in Parkinsons disease (off-label), 1mg three times a day. For the treatment of severe drooling in children aged 3 years and older with chronic neurological disorders. See Electronic medicines compendium for dosing information. |
| 2mg tablet: £123.00 (30) | |||
| as Sialanar® | 320mcg/ml oral solution: £76.80 (60ml) | For the treatment of severe drooling in children aged 3 years and older with chronic neurological disorders. See Electronic medicines compendium for dosing information. |
|
| 1mg/5ml Oral solution: £112.20 (150ml) | For the treatment of severe drooling in children aged 3 years and older with chronic neurological disorders and indicated for use in adults as an add-on therapy in the treatment of peptic ulcer. See Electronic medicines compendium for dosing information. How to manage common side effects. See Reminder Card for Caregivers. Checklist for Healthcare Professionals. See Risk minimisation of anticholinergic side effects. |
||
| Hyoscine hydrobromide as Kwells® | 150mcg tablet: £1.84 (12) | For the treatment of severe drooling in Parkinsons disease (off-label) 150-300mcg three times a day. | |
| 300mcg tablet: £1.84 (12) | |||
| as Scopoderm® | 1.5mg patch: £12.87 (2) | For the treatment of severe drooling of Parkinsons disease (off-label). One patch delivers 1mg hyoscine over 72 hours. 1-2 patches every 72 hours, lower doses can also be used (e.g. ¼ patch every 72 hours). | |
| NHS Somerset classify Trihexyphenidyl is not recommended as per Traffic light guidance. | |||
| Muscle relaxants, peripherally acting neurotoxins | Botulinum toxin A is classified by NHS Somerset as a Red drug (specialist prescribing only) as per Traffic light guidance. | ||