Related guidance:
Opioids Aware (Faculty of Pain Medicine of the Royal College of Anaesthetists)
A resource for patients and healthcare professionals to support prescribing of opioid medicines for pain.
- Opioids are very good analgesics for acute pain and for pain at the end of life but there is little evidence that they are helpful for long term pain.
- A small proportion of people may obtain good pain relief with opioids in the long-term if the dose can be kept low and especially if their use is intermittent (however it is difficult to identify these people at the point of opioid initiation).
- The risk of harm increases substantially at doses above an oral morphine equivalent of 120mg/day, but there is no increased benefit: tapering or stopping high dose opioids needs careful planning and collaboration.
- If a patient has pain that remains severe despite opioid treatment it means they are not working and should be stopped, even if no other treatment is available.
- Chronic pain is very complex and if patients have refractory and disabling symptoms, particularly if they are on high opioid doses, a very detailed assessment of the many emotional influences on their pain experience is essential.
Information for patients
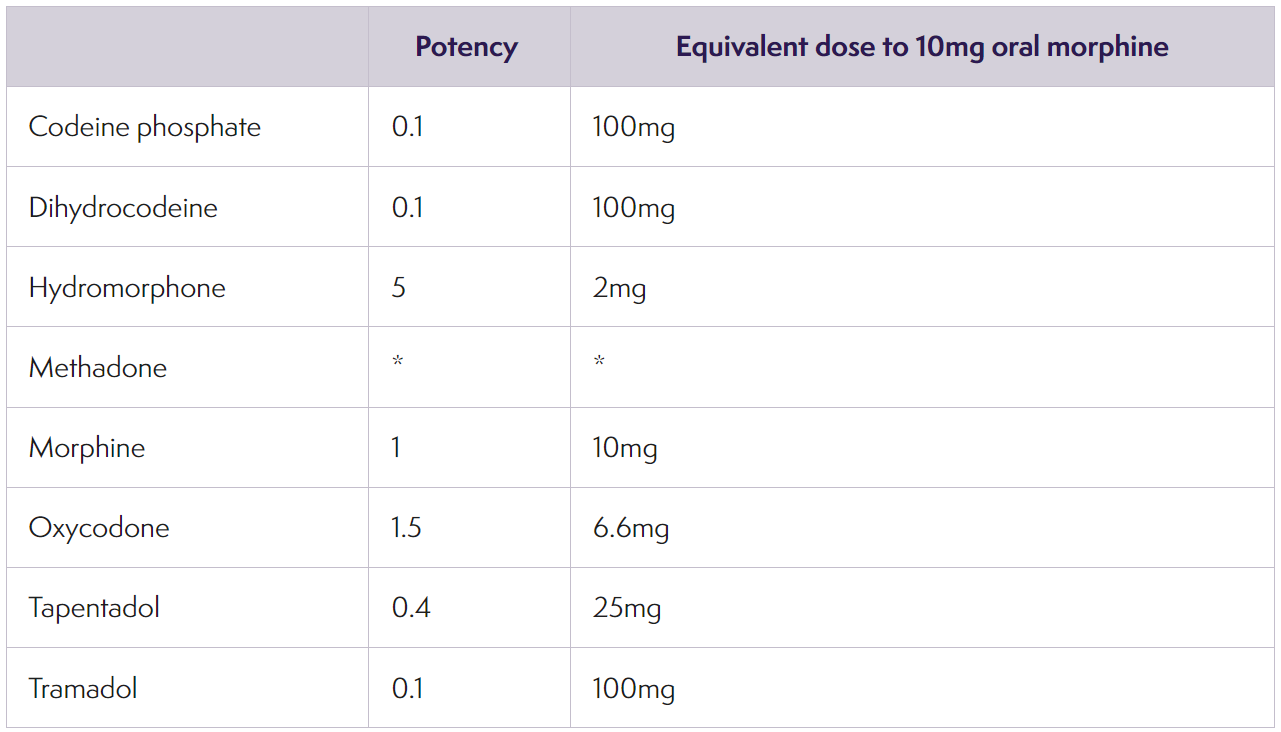
- Approximate equi-analgesic potencies of opioids for oral administration (August 2020)
* The relative potency of methadone depends on the starting dose and the duration of administration. Conversions to and from methadone should always be undertaken with specialist advice
Medication decision aid for those with persistent pain who experience flare-ups (SFT)
- A resource for patients and healthcare professionals to support prescribing for flare-up pain of the muscles, ligaments, or soft tissue. Co-codamol, tramadol and anti-inflammatory medication should only be taken for a short period.
Medication decision aid for those with nerve pain (SFT)
- A resource for patients and healthcare professionals to support prescribing for the management of all nerve type pain, such as stabbing, shooting, or burning pain.
- It is now an offence to drive with any of 17 controlled drugs above a specified level in your blood – this includes illegal and medical drugs.
Prescription medicines include
- amphetamine, for example dexamphetamine or selegiline
- clonazepam
- diazepam
- flunitrazepam
- lorazepam
- methadone
- morphine or opiate and opioid-based drugs, for example codeine, tramadol or fentanyl
- oxazepam
- temazepam
Pain: treatment during pregnancy (SPS January 2022, updated September 2022)
Using opioids for pain relief during pregnancy (SPS August 2022, updated April 2023)
Treating opioid dependence during breastfeeding (SPS August 2023)
Headaches in over 12s: diagnosis and management (CG150 September 2012, updated December 2021)
Medication overuse headache
- Be alert to the possibility of medication overuse headache in people whose headache developed or worsened while they were taking the following drugs for 3 months or more:
- triptans, opioids, ergots or combination analgesic medications on 10 days per month or more or
- paracetamol, aspirin or an NSAID, either alone or any combination, on 15 days per month or more.
Tension-type headache acute treatment
- Consider aspirin, paracetamol or an NSAID for the acute treatment of tension-type headache, taking into account the person’s preference, comorbidities and risk of adverse events.
- Because of the association with Reye’s syndrome, preparations containing aspirin should not be offered to under 16s.
- Do not offer opioids for the acute treatment of tension-type headache.
For aspirin, see NHS Somerset Formulary Antiplatelet drugs
For NSAIDs, see NHS Somerset Formulary Arthritis
Treatments for migraine
- Although there is no cure for migraine, there are treatments that can help. Your healthcare professional should offer you a triptan together with either an NSAID or paracetamol to help relieve migraine. If you prefer to take only 1 drug, they may offer you a triptan, an NSAID, high-dose aspirin or paracetamol. They may also offer you an anti-emetic. All of these drugs are oral drugs. If you are unable to take oral drugs, or they do not work well, you may be offered metoclopramide or prochlorperazine, which are non-oral drugs. You may also be offered a non-oral NSAID or triptan.
- Because of the association with Reye’s syndrome, preparations containing aspirin should not be offered to under 16s.
- Do not offer an ergot or an opioid to treat migraine.
For anti-emetics, see NHS Somerset Formulary Nausea and labyrinth disorders
Prophylactic treatment
- Discuss the benefits and risks of prophylactic treatment for migraine with the person, taking into account the person’s preference, comorbidities, risk of adverse events and the impact of the headache on their quality of life.
- For the prophylaxis of migraine, offer topiramate or propranolol after a full discussion of the benefits and risks of each option. Include in the discussion:
• the potential benefit in reducing migraine recurrence and severity
• the risk of fetal malformations with topiramate
• the risk of reduced effectiveness of hormonal contraceptives with topiramate
• the importance of effective contraception for women and girls of childbearing potential who are taking topiramate (for example, by using medroxyprogesterone acetate depot injection, an intrauterine method or
combined hormonal contraception with a barrier method). Follow the MHRA safety advice on antiepileptic drugs in pregnancy (off-label use of topiramate in children and young people).
For propranolol, see NHS Somerset Formulary Blood pressure conditions
- People with depression and migraine could be at an increased risk of using propranolol for self-harm. Use caution when prescribing propranolol, in line with the Healthcare Safety Investigation Branch’s report on the under-recognised risk of harm from propranolol.
- Consider amitriptyline for the prophylactic treatment of migraine according to the person’s preference, comorbidities and risk of adverse events (off-label use of amitriptyline).
- Do not offer gabapentin for the prophylactic treatment of migraine.
- If both topiramate and propranolol are unsuitable or ineffective, consider a course of up to 10 sessions of acupuncture over 5 to 8 weeks according to the person’s preference, comorbidities and risk of adverse
events. - For people who are already having treatment with another form of prophylaxis and whose migraine is well controlled, continue the current treatment as required.
- Review the need for continuing migraine prophylaxis 6 months after the start of prophylactic treatment.
Rimegepant for preventing migraine Technology appraisal guidance (TA906 July 2023)
- Rimegepant is recommended as an option for preventing episodic migraine in adults who have at least 4 and fewer than 15 migraine attacks per month, only if at least 3 preventative treatments have not worked.
- Stop rimegepant after 12 weeks of treatment if the frequency of migraine attacks does not reduce by at least 50%.
Rimegepant for treating migraine Technology appraisal guidance (TA919 October 2023)
- Rimegepant is recommended as an option for the acute treatment of migraine with or without aura in adults, only if for previous migraines: at least 2 triptans were tried and they did not work well enough or triptans were contraindicated or not tolerated, and nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol were tried but did not work well enough.
Atogepant for preventing migraine Technology appraisal guidance (TA973 May 2024)
- Atogepant is recommended as an option for preventing migraine in adults who have at least 4 migraine days per month, only if at least 3 preventive medicines have failed.
- Stop atogepant after 12 weeks if the frequency of migraines does not reduce by: at least 50% in episodic migraine (defined as fewer than 15 headache days per month), at least 30% in chronic migraine (defined as 15 or more headache days per month, with at least 8 of those having features of migraine).
Migraine (NICE CKS September 2022)
Stages of a migraine attack factsheet
- When agreeing a treatment plan with the person, take into account their concerns and expectations, and discuss:
• the severity of the pain, and its impact on lifestyle, daily activities (including sleep disturbance) and participation
• the underlying cause of the pain and whether this condition has deteriorated
• why a particular pharmacological treatment is being offered
• the benefits and possible adverse effects of pharmacological treatments, taking into account any physical or psychological problems, and concurrent medications
• the importance of dosage titration and the titration process, providing the person with individualised information and advice
• coping strategies for pain and for possible adverse effects of treatment
• non-pharmacological treatments, for example, physical and psychological therapies (which may be offered through a rehabilitation service) and surgery (which may be offered through specialist services). - Consider referring the person to a specialist pain service and/or a condition-specific service at any stage, including at initial presentation and at the regular clinical reviews, if:
• they have severe pain or
• their pain significantly limits their lifestyle, daily activities (including sleep disturbance) and participation or
• their underlying health condition has deteriorated. - Continue existing treatments for people whose neuropathic pain is already effectively managed, taking into account the need for regular clinical reviews.
- When introducing a new treatment, take into account any overlap with the old treatments to avoid deterioration in pain control.
- After starting or changing a treatment, carry out an early clinical review of dosage titration, tolerability and adverse effects to assess the suitability of the chosen treatment.
- Carry out regular clinical reviews to assess and monitor the effectiveness of the treatment. Each review should include an assessment of:
• pain control
• impact on lifestyle, daily activities (including sleep disturbance) and participation
• physical and psychological wellbeing
• adverse effects
• continued need for treatment. - When withdrawing or switching treatment, taper the withdrawal regimen to take account of dosage and any discontinuation symptoms.
All neuropathic pain (except trigeminal neuralgia)
- Offer a choice of amitriptyline, duloxetine, gabapentin or pregabalin as initial treatment for neuropathic pain (except trigeminal neuralgia).
- If the initial treatment is not effective or is not tolerated, offer one of the remaining 3 drugs, and consider switching again if the second and third drugs tried are also not effective or not tolerated.
- Consider tramadol only if acute rescue therapy is needed (not for long-term use).
- Do not start the following to treat neuropathic pain in non-specialist settings, unless advised by a specialist to do so:
• cannabis sativa extract
• capsaicin patch
• lacosamide
• lamotrigine
• levetiracetam
• morphine
• oxcarbazepine
• topiramate
• tramadol (long-term use)
• venlafaxine
For duloxetine and amitriptyline, see NHS Somerset Formulary Depression
For capsaicin see NHS Somerset Formulary Arthritis
For lidocaine medicated plaster, see NHS Somerset Formulary Anaesthesia
Trigeminal neuralgia
- Offer carbamazepine as initial treatment for trigeminal neuralgia. Follow the MHRA safety advice on antiepileptic drugs in pregnancy.
- If initial treatment with carbamazepine is not effective, is not tolerated or is contraindicated, consider seeking expert advice from a specialist and consider early referral to a specialist pain service or a condition-specific service.
- Do not offer gabapentinoids, other antiepileptics, oral corticosteroids or benzodiazepines for managing sciatica
- Do not offer opioids for managing chronic sciatica
Pregabalin for acute and chronic pain in adults (Cochrane Library 2015)
Serotonin Syndrome (Patient May 2019)
Sodium content of cold and flu preparations and painkillers (SPS October 2021)
- Many effervescent and soluble painkillers and some cold and flu preparations contain significant amounts of sodium. The amount of sodium in non-soluble analgesics is insignificant. Orodispersible preparations do not contain significant amounts of sodium.
- Increasing the level of sodium in the body causes an expansion of the extracellular fluid which increases blood pressure. Maintaining steady sodium levels is principally achieved through regulation of excretion through the kidneys. The capacity for renal excretion is lower in the very young and the elderly.
- Increasing long-term intake of dietary sodium has been shown to increase BP across all study populations and age ranges. Prolonged high BP has been associated with stroke, myocardial infarction, heart failure and kidney disease and has also been linked to dementia and premature death.
- Effective management of pain can play an important part in the treatment of agitation and could reduce the number of unnecessary prescriptions for psychotropic drugs in this population.
Controlled drugs – regulations and classification (BNF)
- The Department of Health and the Scottish Government have issued a strong recommendation that the maximum quantity of Schedule 2, 3 or 4 Controlled Drugs prescribed should not exceed 30 days; exceptionally, to cover a justifiable clinical need and after consideration of any risk, a prescription can be issued for a longer period, but the reasons for the decision should be recorded on the patient’s notes. e.g.
Morphine oral MR formulations with twice daily frequency, should not exceed 60 tablets/capsules.
Fentanyl transdermal 72 hourly patches should not exceed 10 patches per month.
Buprenorphine transdermal 7 day patches should not exceed 4 per month.
Buprenorphine transdermal twice weekly patches should not exceed 8 patches per month.
See NHS Somerset Pain management
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Analgesics | |||
| Non-opioid | 500mg tablet: £0.82 (32) | First choice drug in acute and chronic pain. Adult: 500mg-1g every 4-6 hours. Maximum 4g in 24 hours. Recommended as self-care if being used to treat minor ailments. Ensure adequate dose is being used before considering other options. |
|
| 120mg/5ml oral suspension sugar free: £3.76 (100ml) | |||
| 250mg/5ml oral suspension sugar free: £6.64 (200ml) | |||
| NHS Somerset classify Paracetamol 500mg/5ml as not recommended as per Traffic light guidance. | |||
| Non-steroidal anti-inflammatory drugs (NSAIDs) | See NSAIDs in formulary for musculoskeletal pain. | ||
| Antithrombotic drugs, Antiplatelet drugs | See Aspirin in formulary for acute tension-type headache and migraine. | ||
| Topical plant alkaloids | See Capsaicin in formulary for knee or hand osteoarthritis and symptomatic relief of neuralgia. | ||
| Anaesthetics, Local | See Lidocaine in formulary for symptomatic relief of neuropathic pain. | ||
| Opioids | Benzodiazepines and opioids can both cause respiratory depression, which can be fatal if not recognised in time. Only prescribe together if there is no alternative and closely monitor patients for signs of respiratory depression. See MHRA Drug Safety Update (March 2020) for Benzodiazepines and opioids: reminder of risk of potentially fatal respiratory depression. | ||
| Recommendations following a review of the risks of dependence and addiction associated with prolonged use of opioid medicines (opioids) for non-cancer pain. Before prescribing opioids, discuss with the patient the risks and features of tolerance, dependence, and addiction, and agree together a treatment strategy and plan for end of treatment. See MHRA Drug Safety Update (September 2020) for Opioids: risk of dependence and addiction. | |||
| NHS Somerset strongly recommend that prescribers discuss the risk of addiction when initiating new patients on any opioid containing medication and also on review. This discussion should be recorded in the patient notes. Just three days of codeine or dihydrocodeine medicines can lead to addiction. It is recommended that standard paracetamol treatment is prescribed with codeine 15mg tablets if required for breakthrough pain to allow for flexible dosing. |
|||
| Codeine | Codeine should only be used to relieve acute moderate pain in children older than 12 years and only if it cannot be relieved by other painkillers such as paracetamol or ibuprofen alone. Furthermore, a significant risk of serious and life-threatening adverse reactions has been identified in children with obstructive sleep apnoea who received codeine after tonsillectomy or adenoidectomy (or both). Codeine is now contraindicated in all children younger than 18 years who undergo these procedures for obstructive sleep apnoea. See MHRA Drug Safety Update (December 2014) for Codeine for analgesia: restricted use in children because of reports of morphine toxicity | ||
| Codeine is converted to morphine in the liver by the CYP2D6 enzyme. There are many genetic variations of CYP2D6, which affect the extent of this conversion in individuals. People can be classified as: poor; intermediate; extensive; or ultra-rapid metabolisers. Different plasma morphine concentrations in patients’ blood lead not only to different levels of pain relief, but also to a variable and unpredictable risk of side effects due to morphine’s action on the brain and respiratory centre. Poor metabolisers convert very little codeine into morphine and therefore have little or no pain relief. 7–10% of Caucasians are poor metabolisers, but this proportion varies with ethnic origin. Individuals who are ultra-rapid metabolisers or extensive metabolisers have an excessive amount of morphine in their blood, which can lead to severe side effects due to its effects on the brain and on breathing. Prevalence of ultra-rapid metabolisers also varies by ethnic origin: it is less common in northern European countries (including the UK) and is more common in Spain, Italy, Greece, Africa, and the Middle East. |
|||
| 15mg tablet: £0.79 | First-choice opiate for short-term treatment of acute moderate pain. Adult: 30–60 mg every 6 hours as required for maximum 3 days. Maximum 240mg in 24 hours. For diarrhoea. Adult: 15–60 mg 3–4 times a day. |
||
| 30mg tablet: £0.99 | |||
| 60mg tablet: £1.91 | |||
| 15mg/5ml linctus sugar free: £1.98 (200ml) | |||
| Co-codamol as Emcozin® | 30mg/500mg tablet: £2.94 (100) | 30/500–60/1000 mg every 4–6 hours as required; maximum 240/4000 mg per day. | |
| NHS Somerset classify Co-codamol 8/500 or 15/500 and Co-dydramol as not recommended as per Traffic light guidance. | |||
| NHS Somerset classify Co-proxamol as not recommended as no longer licensed due to safety concerns as per Traffic light guidance. | |||
| Tramadol | Tramadol is metabolised by the liver enzyme CYP2D6. If a patient has a deficiency or is completely lacking this enzyme an adequate analgesic effect may not be obtained. Estimates indicate that up to 7% of the Caucasian population may have this deficiency. However, if the patient is an ultra-rapid metaboliser there is a risk of developing side effects of opioid toxicity even at commonly prescribed doses. Lowers seizure threshold and risk of serotonin syndrome, a potentially life-threatening condition, has been reported in patients receiving tramadol in combination with other serotonergic agents or tramadol alone. |
||
| 50mg capsule: £0.88 (30) | Tramadol may be appropriate to consider as an alternative to Codeine where its efficacy or tolerability is poor. Adult: 50-100mg every 4-6 hours. Maximum 400mg in 24 hours. For neuropathic pain when acute rescue therapy is needed (not for long-term use). |
||
| 50mg soluble tablet sugar free: £13.33 (100) | Contains less than 1 mmol sodium (23 mg) per dispersible tablet, that is to say essentially 'sodium-free' | ||
| 50mg/5ml oral solution sugar free: £4.50 (100ml) | |||
| as Marol® | 100mg modified release tablet: £6.94 (60) | For patients with long term chronic pain responsive to tramadol but who suffer significant side effects from the immediate release capsules a modified release product may be prescribed. Adult: twice daily formulation. Maximum 400mg in 24 hours. |
|
| 150mg modified release tablet: £10.39 (60) | |||
| 200mg modified release tablet: £14.19 (60) | |||
| as Tramulief® | 100mg modified release tablet: £6.98 (60) | ||
| 150mg modified release tablet: £10.48 (60) | |||
| 200mg modified release tablet: £14.28 (60) | |||
| as Tradorec XL® | 100mg modified release tablet: £14.10 (30) | Adult: once daily formulation. Maximum 400mg in 24 hours. | |
| 200mg modified release tablet: £14.98 (30) | |||
| 300mg modified release tablet: £22.47 (30) | |||
| NHS Somerset classify Tramacet® (paracetamol and tramadol) as not recommended as per Traffic light guidance. | |||
| Morphine as Actimorph® | 1mg orodispersible tablet sugar free: £2.00 (56) | First line for initial dose titration and breakthrough pain every 4-6 hours. Use of immediate release morphine preparations can provide effective symptomatic relief and use of such regimens may be justified when : • the pain is intermittent and short-lived; • pain intensity varies significantly: use of regimens including immediate release preparations allows flexibility to reduce dose on days when pain is or is expected to be less severe; or • background pain is well controlled with modified release preparations but the patient has infrequent, short-lived episodes of increased pain. Modified release opioids administered at regular intervals may be more appropriate for patients with persistent pain throughout the day and night. Can be taken with or without food. The tablet disperses rapidly in the mouth and is then swallowed. Alternatively, for special population such as children or patients with difficulties in swallowing, the tablet may be placed in a spoon with the addition of a small quantity of water until sufficient dispersion to allow ingestion. Actimorph is a CD schedule 2. |
|
| 2.5mg orodispersible tablet sugar free: £2.50 (56) | |||
| 5mg orodispersible tablet: £3.50 (56) | |||
| 10mg orodispersible tablet: £4.75 (56) | |||
| 20mg orodispersible tablet: £9.50 (56) | |||
| 30mg orodispersible tablet: £13.00 (56) | |||
| as Sevredol® | 10mg tablet: £5.31 (56) | Use for initial dose titration and breakthrough pain every 4 hours. Sevredol is a CD schedule 2. |
|
| 20mg tablet: £10.61 (56) | |||
| 50mg tablet: £28.02 (56) | |||
 |
|||
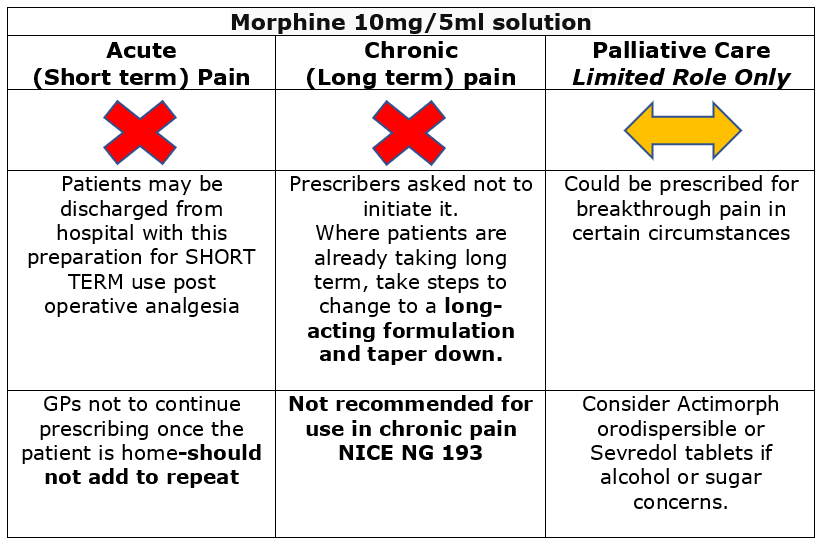
| 10mg/5ml oral solution: £4.03 (300ml) | Use for initial dose titration and breakthrough pain every 4 hours. Morphine solution 10mg/5ml is a CD schedule 5, unlike its equivalent strength of tablets or capsules which are schedule 2. It is therefore not subject to the same rules of prescribing, recording and storage as the solid dose forms. Do not confuse with Oramorph Concentrated oral solution which is 100mg/5ml (120ml) and is designed to be measured with the accompanying dropper. There are safety concerns associated with the use of oral morphine sulfate solution. It is a high-risk medication with increased risk of overdose and death. Coroners have raised concerns about its safety. Please note that oral morphine sulfate solution contains alcohol and sugar. Actimorph OR Sevredol may be preferable alternatives if a short acting oral morphine formulation is required. |
||
| as Zomorph® | 10mg modified-release capsule: £3.47 (60) | NHS Somerset classify as an Amber drug for management of neuropathic pain recommended by a specialist pain service or a condition specific service as per traffic light guidance. Prescribe 12-hourly. Modified-release morphine is a CD schedule 2. Zomorph capsules can be opened and sprinkled on semi-solid food (e.g. yoghurt) or given in water via NG tube. Do not crush. |
|
| 30mg modified-release capsule: £8.30 (60) | |||
| 60mg modified-release capsule: £16.20 (60) | |||
| 100mg modified-release capsule: £21.80 (60) | |||
| 200mg modified-release capsule: £43.60 (60) | |||
| as Morphgesic SR® | 10mg modified-release tablet: £3.85 (60) | ||
| 30mg modified-release tablet: £9.24 (60) | |||
| 60mg modified-release tablet: £18.04 (60) | |||
| 100mg modified-release tablet: £28.54 (60) | |||
| 10mg/1ml solution for injection ampoule: £5.22 (10) | For palliative care as per Wessex palliative care handbook and topical application in combination with intrasite gel as per SFT management of malignant fungating wounds guideline. | ||
| Fentanyl | Reports of unintentional overdose of fentanyl due to dosing errors, accidental exposure, and exposure of the patch to a heat source. Fentanyl is a potent opioid analgesic and should be used only in patients who have previously tolerated opioids. See MHRA Drug Safety Update (September 2008) for Fentanyl patches: serious and fatal overdose from dosing errors, accidental exposure, and inappropriate use. | ||
| as Opiodur® | 12micrograms/hour transdermal patch: £5.64 (5) | For management of severe chronic pain that requires continuous long term opioid administration, second line after oral morphine. Fentanyl is a CD schedule 2. The fentanyl patch should be replaced every 72 hours and new patch should be applied to a different skin site. Do not cut the patch. Caution is advised when fentanyl is co-administered with medicinal products that affect the serotonergic neurotransmitter systems. The development of a potentially life-threatening serotonin syndrome may occur with the concomitant use of serotonergic active substances such as Selective Serotonin Re-uptake Inhibitors (SSRIs) and Serotonin Norepinephrine Re-uptake Inhibitors (SNRIs), and with active substances that impair metabolism of serotonin (including Monoamine Oxidase Inhibitors [MAOIs]). Increased body temperature, exposure of patches to external heat sources, and concomitant use of CYP3A4 inhibitors may lead to potentially dangerous rises in serum fentanyl levels. Concomitant use of other CNS depressants might also potentiate adverse effects from fentanyl. |
|
| 25micrograms/hour transdermal patch: £8.07 (5) | |||
| 50micrograms/hour transdermal patch: £15.09 (5) | |||
| 75micrograms/hour transdermal patch: £21.05 (5) | |||
| 100micrograms/hour transdermal patch: £25.94 (5) | |||
| as Matrifen® | 12micrograms/hour transdermal patch:£7.52 (5) | ||
| 25micrograms/hour transdermal patch: £10.76 (5) | |||
| 50micrograms/hour transdermal patch: £20.12 (5) | |||
| 75micrograms/hour transdermal patch: £28.06 (5) | |||
| 100micrograms/hour transdermal patch: £34.59 (5) | |||
| as Mezolar® | 12micrograms/hour transdermal patch: £7.53 (5) | ||
| 25micrograms/hour transdermal patch: £10.77 (5) | |||
| 37.5micrograms/hour transdermal patch: £15.46 (5) | |||
| 50micrograms/hour transdermal patch: £20.13 (5) | |||
| 75micrograms/hour transdermal patch: £28.07 (5) | |||
| 100micrograms/hour transdermal patch: £34.60 (5) | |||
| as Fencino® | 12micrograms/hour transdermal patch: £8.46 (5) | ||
| 25micrograms/hour transdermal patch: £12.10 (5) | |||
| 50micrograms/hour transdermal patch: £22.62 (5) | |||
| 75micrograms/hour transdermal patch: £31.54 (5) | |||
| 100micrograms/hour transdermal patch: £38.88 (5) | |||
| as Effentora® | 100microgram buccal tablet sugar free: £139.72 | NHS Somerset classify as an Amber drug for the management of breakthrough pain in adult patients using opioid therapy for chronic cancer pain for whom other short acting opioids e.g. oral morphine are unsuitable and Black (not recommended) for all other indications as per Traffic light guidance. Switching from other fentanyl containing products must not occur at a 1:1 ratio because of different absorption profiles. |
|
| 200microgram buccal tablet sugar free: £139.72 | |||
| 400microgram buccal tablet sugar free: £139.72 | |||
| 600microgram buccal tablet sugar free: £139.72 | |||
| 800microgram buccal tablet sugar free: £139.72 | |||
| as Abstral® | 100microgram sublingual tablet sugar free: £49.99 (10) | ||
| 200microgram sublingual tablets sugar free: £49.99 (10) | |||
| 300microgram sublingual tablets sugar free: £49.99 (10) | |||
| 400microgram sublingual tablets sugar free: £49.99 (10) | |||
| 600microgram sublingual tablets sugar free: £49.99 (10) | |||
| 800microgram sublingual tablets sugar free: £49.99 (10) | |||
| NHS Somerset classify Fentanyl nasal spray as not recommended as per Traffic light guidance. | |||
| Buprenorphine | Opioid receptor partial agonist for moderate to severe chronic pain which does not respond to non-opioid analgesics. Buprenorphine is a CD schedule 3. The buprenorphine patch should be replaced when specified as per brand and new patch should be applied to a different skin site. Do not cut the patch. Increased body temperature, exposure of patches to external heat sources may lead to potentially dangerous rises in serum buprenorphine levels. |
||
| as Rebrikel® | 5micrograms/hour transdermal patch: £5.25 (4) | Replace patch every 7 days. Bunov 20mcg is bioequivalent to Butrans. 5mcg and 10mcg strengths were subject to biowaiver. |
|
| 10micrograms/hour transdermal patch: £9.43 (4) | |||
| 20micrograms/hour transdermal patch: £17.19 (4) | |||
| as Bunov® | 5micrograms/hour transdermal patch: £5.54 (4) | ||
| 10micrograms/hour transdermal patch: £9.94 (4) | |||
| 20micrograms/hour transdermal patch: £18.10 (4) | |||
| as Reletrans® | 5micrograms/hour transdermal patch: £6.34 (4) |
||
| 10micrograms/hour transdermal patch: £11.36 (4) | |||
| 15micrograms/hour transdermal patch: £17.70 (4) | |||
| 20micrograms/hour transdermal patch: £20.06 (4) | |||
| as Butec® | 5micrograms/hour transdermal patch: £7.92 (4) | ||
| 10micrograms/hour transdermal patch: £14.20 (4) | |||
| 15micrograms/hour transdermal patch: £22.12 (4) | |||
| 20micrograms/hour transdermal patch: £25.86 (4) | |||
| as Hapoctasin® | 35micrograms/hour transdermal patch: £9.48 (4) | Replace patch every 72 hours. | |
| 52.5micrograms/hour transdermal patch: £14.23 (4) | |||
| 70micrograms/hour transdermal patch: £18.96 (4) | |||
| as Relevtec® | 35micrograms/hour transdermal patch: £11.06 (4) | Replace patch every 96 hours. | |
| 52.5micrograms/hour transdermal patch: £16.60 (4) | |||
| 70micrograms/hour transdermal patch: £22.12 (4) | |||
| 2mg sublingual tablets sugar free: £1.44 (7) | NHS Somerset classify as an Amber drug for the treatment of drug addiction: Public health commissioned drug addiction service - Public health commission some GP practices to prescribe buprenorphine for drug addiction but not all. Not recommended for primary care prescribing by GP practices when not part of the commissioned service. Not recommended for other indications. |
||
| 8mg sublingual tablets sugar free: £3.89 (7) | |||
| NHS Somerset classify Temgesic® as not recommended as per Traffic light guidance. | |||
| Methadone | 1mg/ml oral solution sugar free: £1.04 (100ml) | NHS Somerset classify as an Amber drug for the treatment of drug addiction: Public health commissioned drug addiction service - Public health commission some GP practices to prescribe Methadone for drug addiction but not all. Not recommended for primary care prescribing by GP practices when not part of the commissioned service. NHS Somerset classify as an Amber drug for the treatment of treatment resistant pain: only for primary care prescribing by GP practices on the advice and supervision of suitable specialists in pain management. Do not confuse with the 20mg/ml concentration. |
|
| NHS Somerset classify parenteral methadone as not recommended as per Traffic light guidance. | |||
| Oxycodone | Oxycodone should only be used as a second-line strong opioid, if morphine is not suitable or cannot be tolerated. The specialist pain or palliative care team should be consulted for advice in cases of complex pain management. See CQC (July 2016) for Safer Use of Controlled Drugs – Preventing Harm From Oral Oxycodone Medicines | ||
| as Oxyact® | 5mg tablet: £5.15 (56) | Adult: 4-6 hourly dose. | |
| 10mg tablet: £10.29 (56) | |||
| 20mg tablet: £20.57 (56) | |||
| as Shortec® | 5mg capsule: £6.86 (56) | ||
| 10mg capsule: £13.72 (56) | |||
| 20mg capsule: £27.43 (56) | |||
| 5mg/5ml oral solution sugar free: £8.09 (250ml) | Do not confuse with 10mg/ml concentrate oral solution. | ||
| as Oxylan® | 5mg modified-release tablet: £2.66 | Adult: 12 hourly dose. | |
| 10mg modified-release tablet: £5.32 (56) | |||
| 15mg modified-release tablet: £7.82 (56) | |||
| 20mg modified-release tablet: £9.84 (56) | |||
| 30mg modified-release tablet: £16.80 (56) | |||
| 40mg modified-release tablet: £21.17 (56) | |||
| 60mg modified-release tablet: £32.43 (56) | |||
| 80mg modified-release tablet: £42.62 (56) | |||
| as Oxeltra® | 5mg modified-release tablet: £3.13 | ||
| 10mg modified-release tablet: £6.26 (56) | |||
| 15mg modified-release tablet: £9.53 (56) | |||
| 20mg modified-release tablet: £12.52 (56) | |||
| 30mg modified-release tablet: £19.06 (56) | |||
| 40mg modified-release tablet: £25.05 (56) | |||
| 60mg modified-release tablet: £38.12 (56) | |||
| 80mg modified-release tablet: £50.10 (56) | |||
| as Oxypro® | 5mg modified-release tablet: £3.13 | ||
| 10mg modified-release tablet: £6.26 (56) | |||
| 15mg modified-release tablet: £9.53 (56) | |||
| 20mg modified-release tablet: £12.52 (56) | |||
| 30mg modified-release tablet: £19.06 (56) | |||
| 40mg modified-release tablet: £25.05 (56) | |||
| 60mg modified-release tablet: £38.12 (56) | |||
| 80mg modified-release tablet: £50.10 (56) | |||
| NHS Somerset classify Oxycodone and naloxone as not recommended as per Traffic light guidance. | |||
| NHS Somerset classify Pentazocine as not recommended as per Traffic light guidance. | |||
| NHS Somerset classify Pethidine as not recommended for moderate to severe pain and Red (specialist prescribing only) for Obsteric analgesia and Peri-operative pain as per Traffic light guidance. | |||
| Tapentadol | Tapentadol may increase seizure risk in patients taking other medicines that lower seizure threshold, for example, antidepressants and antipsychotics. Serotonin syndrome has also been reported when tapentadol is used in combination with serotoninergic antidepressants. See MHRA (January 2019) for Tapentadol: risk of seizures and reports of serotonin syndrome when co-administered with other medicines. | ||
| as Palexia® | 50mg tablet: £12.46 | For relief of moderate to severe pain in adults as an alternative to oxycodone. Adult: 4-6 hourly dose. |
|
| 75mg tablet: £18.68 | |||
| 20mg/ml oral solution sugar free: £17.80 (100ml) | |||
| as Tapimio® | 50mg modified-release capsule: £10.59 (28) £21.17 (56) | Adult: 12 hourly dose. | |
| 100mg modified-release capsule: £42.35 (56) | |||
| 150mg modified-release capsule: £63.52 (56) | |||
| 200mg modified-release capsule: £84.69 (56) | |||
| 250mg modified-release capsule: £105.87 (56) | |||
| Alfentanil as Rapifen® | 1mg/2ml solution for injection ampoule: £6.34 (10) | NHS Somerset classify as Amber for use in palliative care patients with chronic kidney disease (eGFR <30ml/min), or severe acute renal impairment as per shared care protocol and Red for all other indications. | |
| NHS Somerset classify Dipipanone and cyclizine as not recommended as per Traffic light guidance. | |||
| Management of opioid overdosage may require use of Naloxone See Anaesthesia and Emergency treatment of poisoning in formulary. | |||
| Triptans | Treatment of acute migraine | ||
| Sumatriptan | 50mg tablet: £1.15 (6) | Sumatripan is the most cost-effective triptan. Adult: Initially 50-100mg orally for 1 dose, followed by 50-100mg after at least 2 hours if required, to be taken only if migraine recurs (patient not responding to initial dose should not take second dose for same attack): maximum 300mg per day. Evidence suggests little additional benefit from doses above 50mg. A trial of oral is recommended prior to initiation of injectable formulations. |
|
| 100mg tablet: £1.38 (6) | |||
| as Imigran® | 10mg/0.1ml nasal spray unit dose: £14.16 (2) | Adult: Initially 10–20 mg, to be administered into one nostril, followed by 10–20 mg after at least 2 hours if required, to be taken only if migraine recurs (patient not responding to initial dose should not take second dose for same attack); maximum 40 mg per day. | |
| 20mg/0.1ml nasal spray unit dose: £42.47 (6) | |||
| 6mg/0.5ml solution for injection pre-filled disposable device: £45.00 (2) | Adult: Intially 6mg subcutaneously for 1 dose, followed by 6mg after at least an hour if required, to be taken only if migraine recurs (patient not responding to initial dose should not take second dose for same attack), dose to be administered using an auto-injector; not for intravenous injection which may cause coronary vasospasm and angina; maximum 12mg a day. Prescribe pack with device and then refill pack to reduce carbon footprint.  |
||
| as Imigran Subject® | 6mg/0.5ml solution for injection pre-filled syringes with device: £50.96 (2) | ||
| 6mg/0.5ml solution for injection syringe refill: £48.49 (2) | |||
| Frovatriptan | 2.5mg tablet: £13.16 (6) | Adult: 2.5mg dose to be taken as soon as possible after onset, followed by 2.5mg after 2 hours if required, dose to be taken only if migraine recurs (patient not responding to initial dose should not take second dose for same attack); maximum 5mg per day. | |
| Naratriptan | 2.5mg tablet: £1.79 (6), £3.58 (12) | Adult: 2.5mg, followed by 2.5mg after 4 hours if required, to be taken only if migraine recurs (patient not responding to initial dose should not take second dose for same attack); maximum 5mg per day. | |
| Zolmitriptan | 2.5mg tablet: £11.72 (6), £23.42 (12) | Adult: 2.5mg, followed by 2.5mg after at least 2 hours if required, dose to be taken only if migraine recurs then increased if necessary to 5mg, dose to be taken for subsequent attacks in patients not achieving satisfactory relief with 2.5mg dose; maximum 10mg per day. There is no evidence that orodispersible formulations have a faster onset of action than conventional tablets. |
|
| 5mg tablet: £36.00 (6) | |||
| 2.5 mg orodispersible tablet sugar free: £2.33 (6) | |||
| 5mg orodispersible tablet sugar free: £15.47 (6) | |||
| Calcitonin gene-related peptide receptor antagonist | Rimegepant as Vydura® | 75mg oral lyophilisates sugar free: £25.80 (2), £103.20 (8) | For preventing episodic migraine in adults who have at least 4 and fewer than 15 migraine attacks per month, only if at least 3 preventative treatments have not worked as per traffic light guidance. Adult: 75 mg once daily on alternate days. Stop rimegepant after 12 weeks of treatment if the frequency of migraine attacks does not reduce by at least 50%. For the acute treatment of migraine with or without aura in adults, only if for previous migraines: at least 2 triptans were tried and they did not work well enough or triptans were contraindicated or not tolerated, and nonsteroidal anti-inflammatory drugs and paracetamol were tried but did not work well enough. Adult: 75 mg once daily if required. Avoid in severe renal and hepatic impairment. |
| Atogepant as Aquipta® | 10mg tablet: £182.16 | For preventing migraine in adults who have at least 4 migraine days per month, only if at least 3 preventive medicines have failed. Stop atogepant after 12 weeks if the frequency of migraines does not reduce by: at least 50% in episodic migraine (defined as fewer than 15 headache days per month), at least 30% in chronic migraine (defined as 15 or more headache days per month, with at least 8 of those having features of migraine). Adult: 60 mg once daily. Reduce dose to 10 mg once daily with concurrent use of potent CYP3A4 inhibitors, OATP inhibitors, or telmisartan. Reduce dose to 10 mg once daily if creatinine clearance less than 30 mL/minute. Avoid in severe impairment (risk of increased exposure). |
|
| 60mg tablet: £182.16 | |||
| Human monoclonal antibody | NHS Somerset classify Eptinezumab for migraine prophylaxis as Red (specialist prescribing only) as per Traffic light guidance. | ||
| NHS Somerset classify Fremanezumab for chronic migraine prophylaxis as Red (specialist prescribing only) as per Traffic light guidance. | |||
| NHS Somerset classify Galcanezumab for migraine prophylaxis as Red (specialist prescribing only) as per Traffic light guidance. | |||
| Muscle relaxants, peripherally acting neurotoxins (botulinum toxins) | NHS Somerset classify Botulinum toxin type A for chronic migraine prophylaxis as Red (specialist prescribing only) as per Traffic light guidance. | ||