Related guidance:
Osteoarthritis in over 16s: diagnosis and management NICE guideline (NG226 October 2022)
- Diagnose osteoarthritis clinically without imaging in people who are 45 or over and have activity-related joint pain and have either no morning joint-related stiffness or morning stiffness that lasts no longer than 30 minutes.
- Do not routinely use imaging to diagnose osteoarthritis unless there are atypical features or features that suggest an alternative or additional diagnosis. See figure 1 below for visual summary on the management of osteoporosis.
- Advise people where they can find further information on osteoarthritis, how it develops including flares, progression over time and information that challenges common misconceptions about the condition, specific types of exercise, managing their symptoms, how to access additional sources of information and support after consultations and benefits and limitations of treatment.
VersusArthritis osteoarthritis patient information booklet
- Offer therapeutic exercise tailored to their needs for example local muscle strengthening and general aerobic fitness.
- Consider supervised therapeutic exercise sessions and advise people with osteoarthritis that joint pain may increase when they start therapeutic exercise. Doing regular and consistent exercise, even though this may initially cause pain or discomfort, will be beneficial for joints and long-term adherence to an exercise plan increases its benefits by reducing pain and increasing functioning and quality of life. Consider combining therapeutic exercise with an education programme or behaviour change approaches in a structured treatment package.
- Advise people with osteoarthritis who are living with overweight or obesity that weight loss will improve their quality of life and physical function and reduce pain. Support them to choose a weight loss goal and explain that any amount of weight loss is likely to be beneficial, but losing 10% of their body weight is likely to be better than 5%. For guidance and information on weight management, including recommended interventions to support weight loss, see NICE’s topic page on obesity.
- Only consider manual therapy such as manipulation, mobilisation or soft tissue techniques for people with hip or knee osteoarthritis and alongside therapeutic exercise. There is not enough evidence to support its use alone for managing osteoarthritis.
- Do not offer acupuncture or dry needling to manage osteoarthritis.
- Do not offer any of the following electrotherapy treatments to people with osteoarthritis because there is insufficient evidence of benefit. These include transcutaneous electrical nerve stimulation (TENS), ultrasound therapy, interferential therapy, laser therapy, pulsed short-wave therapy and neuromuscular electrical stimulation (NMES).
- Consider walking aids such as walking sticks for people with lower limb osteoarthritis.
- Do not routinely offer insoles, braces, tape, splints or supports to people with osteoarthritis unless there is joint instability or abnormal biomechanical loading and therapeutic exercise is ineffective or unsuitable without the addition of an aid or device and the addition of an aid or device is likely to improve movement and function.
- If pharmacological treatments are needed to manage osteoarthritis, use them alongside non-pharmacological treatments and to support therapeutic exercise at the lowest effective dose for the shortest possible time.
- Offer a topical non-steroidal anti-inflammatory drug (NSAID) to people with knee osteoarthritis and consider a topical NSAID for people with osteoarthritis that affects other joints.
- If topical medicines are ineffective or unsuitable, consider an oral NSAID for people with osteoarthritis and take account of potential gastrointestinal, renal, liver and cardiovascular toxicity and any risk factors including age, pregnancy, current medication and comorbidities.
- Offer a gastroprotective treatment (such as a proton pump inhibitor) for people with osteoarthritis while they are taking an NSAID. See Gastric acid disorders and ulceration in formulary.
- Do not routinely offer paracetamol or weak opioids unless they are only used infrequently for short-term pain relief and all other pharmacological treatments are contraindicated, not tolerated or ineffective. There is no strong evidence of benefit for paracetamol and opioids are associated with dependence and withdrawal symptoms. See Pain in formulary.
- Do not offer glucosamine or strong opioids to people to manage osteoarthritis. There is no strong evidence of benefit for glucosamine and the risks of strong opioids outweigh the benefits.
- Review with the person whether to continue treatment and base frequency of reviews on clinical need.
- Do not offer intra-articular hyaluronan injections to manage osteoarthritis.
- Consider intra-articular corticosteroid injections when other pharmacological treatments are ineffective or unsuitable, or to support therapeutic exercise. These only provide short-term relief (2 to 10 weeks).
- Consider patient-initiated follow-up for most people with osteoarthritis.
- Consider planned follow-up for people with osteoarthritis when their individual needs and preferences suggest that this is necessary, taking into account treatments or interventions that need monitoring, their ability to seek help for themselves, their occupation and activities and the severity of their symptoms or functional limitations.
- People with multiple long-term conditions are likely to benefit from a tailored approach in line with NICE’s guideline on multimorbidity.
- Advise people with osteoarthritis to seek follow-up if planned management is not working within an agreed follow-up time or they are having difficulties with the agreed approaches.
- Do not routinely use imaging for follow-up or to guide non-surgical management of osteoarthritis.
- Consider referring people with hip, knee or shoulder osteoarthritis for joint replacement if joint symptoms such as pain, stiffness, reduced function or progressive joint deformity are substantially impacting their quality of life and non-surgical management for example therapeutic exercise, weight loss, pain relief is ineffective or unsuitable.
- Use clinical assessment when deciding to refer someone for joint replacement, instead of systems that numerically score severity of disease.
- Do not exclude people with osteoarthritis from referral for joint replacement because of age, sex or gender, smoking, comorbidities, overweight or obesity based on measurements such as body mass index (BMI). Risks of joint replacement can vary depending on these factors.
- Do not offer arthroscopic lavage or debridement to people with osteoarthritis.
Rheumatoid arthritis in adults: management NICE guideline (NG100 July 2018, updated October 2020)
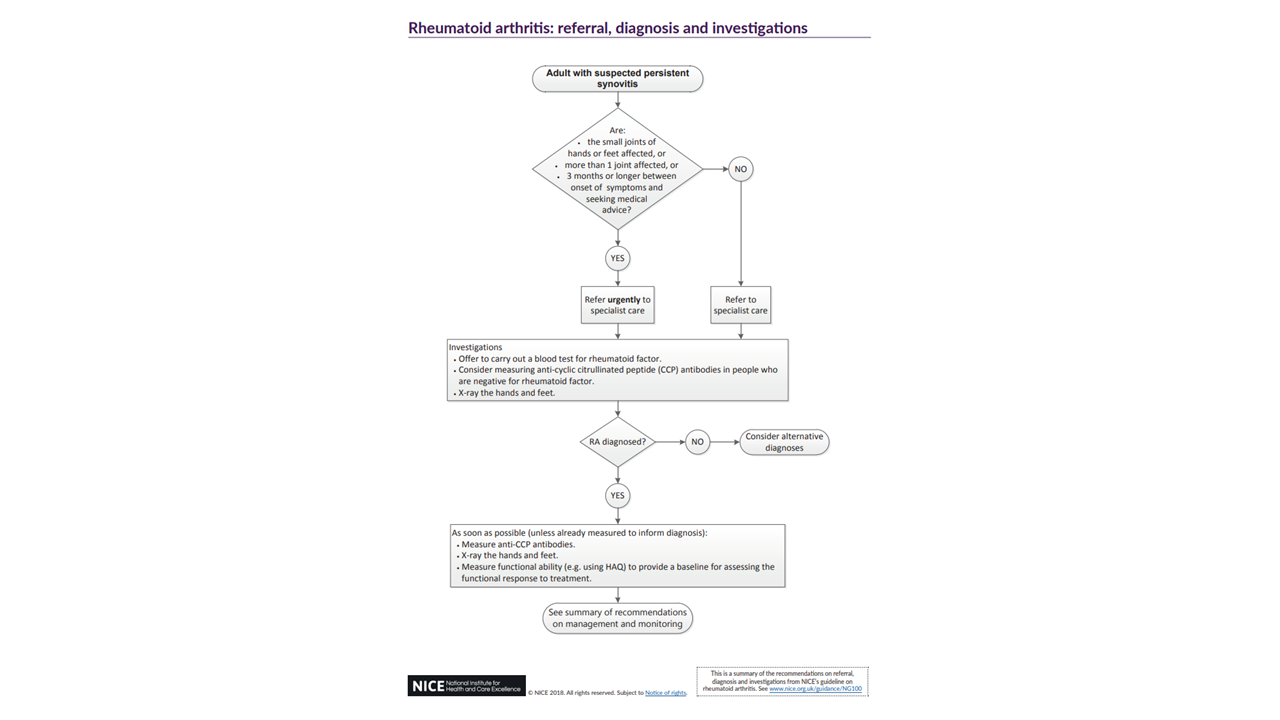
- Refer for specialist opinion any adult with suspected persistent synovitis of undetermined cause. Refer urgently if the small joints of the hands or feet are affected, more than one joint is affected or there has been a delay of 3 months or longer between onset of symptoms and seeking medical advice. See Figure 2 below for rheumatoid arthritis: referral, diagnosis and investigations algorithm.
- Advise seeking rapid access to specialist care if disease worsens or they have a flare.
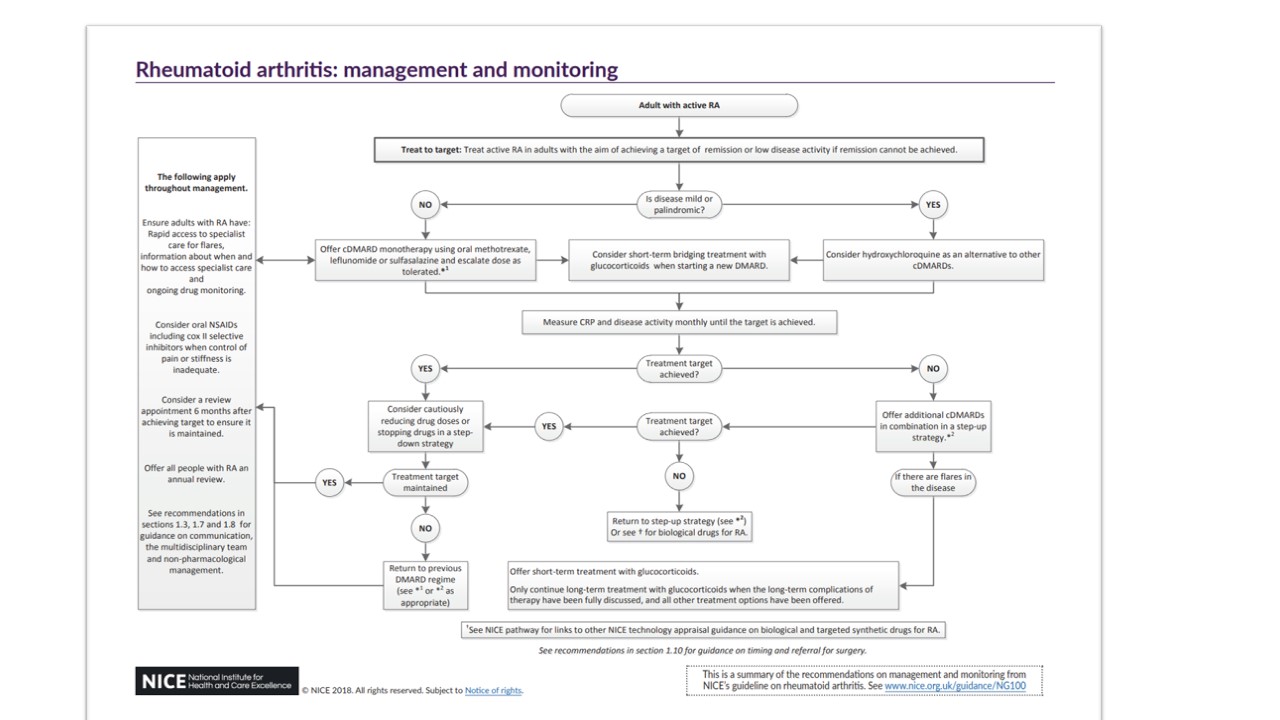
- Treat active rheumatoid arthritis (RA) in adults with the aim of achieving a target of remission or low disease activity if remission cannot be achieved (treat-to-target), which may involve trying multiple conventional disease modifying anti-rheumatic drugs (cDMARDs) and biological DMARDs with different mechanisms of action, one after the other. See Figure 3 below for rheumatoid arthritis: management and monitoring algorithm.
- Explain the risks and benefits of treatment options to adults with RA.
- Offer information to improve understanding of the condition and its management.
VersusArthritis rheumatoid arthritis patient information booklet
- Offer first-line treatment with cDMARD monotherapy using oral methotrexate, leflunomide or sulfasalazine as soon as possible and ideally within 3 months of onset of persistent symptoms. Consider hydroxychloroquine for first-line treatment as an alternative to oral methotrexate, leflunomide or sulfasalazine for mild or palindromic disease and escalate dose as tolerated.
- Consider short-term bridging treatment with glucocorticoids (oral, intramuscular or intra-articular) when starting a new cDMARD. See oral Corticosteriods in formulary.
For Steroid Emergency Card see NHS Somerset Formulary Corticosteriod responsive conditions.
- Offer additional cDMARDs (oral methotrexate, leflunomide, sulfasalazine or hydroxychloroquine) in combination in a step-up strategy when the treatment target (remission or low disease activity) has not been achieved despite dose escalation.
- Offer short-term treatment with glucocorticoids for managing flares in adults with recent-onset or established disease to rapidly decrease inflammation.
- In adults with established RA, only continue long-term treatment with glucocorticoids when the long-term complications of glucocorticoid therapy have been fully discussed, and all other treatment options (including biological and targeted synthetic DMARDs) have been offered.
- Consider NSAIDs, including cox 2 inhibitor NSAIDs, for symptom control when control of pain or stiffness is inadequate. Analgesics other than NSAIDs are no longer recommended, however if a person with RA needs to take low-dose aspirin, other treatments should be considered before adding an NSAID if pain relief is ineffective or insufficient. See Pain in formulary.
- Adults with RA should have rapid access to specialist care for flares, ongoing drug monitoring and an annual review to assess disease activity and damage, measure functional ability, check for the development of comorbidities, such as hypertension, ischaemic heart disease, osteoporosis and depression, assess symptoms that suggest complications, such as vasculitis and disease of the cervical spine, lung or eyes, organise appropriate cross referral within the multidisciplinary team and assess the need for referral for surgery.
- Summary of NICE guidance for recognition and referral of Spondyloarthritis Msk?… Think SpA! (East Sussex Healthcare NHS Trust leaflet updated April 2020)
- Offer information to improve understanding of the condition and its management.
VersusArthritis back pain patient information booklet
VersusArthritis Ankylosing spondylitis and related conditions patient information booklet.
- Ensure people with spondyloarthritis have access to specialist care during flares for self-care (exercises, stretching and joint protection), pain and fatigue management, adverse effects and potential changes to medicines, managing the impact on daily life and ability to work.
- Offer NSAIDs at the lowest effective dose to people with pain associated with axial spondyloarthritis. Consider hydrotherapy as an adjunctive therapy to manage pain and maintain or improve function for people with axial spondyloarthritis.
- Consider a referral to a specialist therapist (physiotherapist, occupational therapist, hand therapist, orthotist or podiatrist) for people with spondyloarthritis who have difficulties with any of their everyday activities to assess needs, provide advice about physical aids and review changing needs.
- Biological DMARDs are options for treating severe active ankylosing spondylitis in adults whose disease has
responded inadequately to, or who cannot tolerate NSAIDs. - For people with psoriatic arthritis and other peripheral spondyloarthritides, consider local corticosteroid injections as monotherapy for nonprogressive monoarthritis and cDMARDs to people with peripheral polyarthritis, oligoarthritis, persistent or progressive monoarthritis associated with peripheral spondyloarthritis. If a cDMARD taken at the maximum tolerated dose for at least 3 months does not provide adequate relief from symptoms, consider switching to or adding another cDMARD. Consider NSAIDs as an adjunct to cDMARDs or biological DMARDs to manage symptoms.
- If NSAIDs do not provide adequate relief from symptoms, consider steroid injections (local or intramuscular) or short-term oral steroid therapy as an adjunct to cDMARDs or biological DMARDs to manage symptoms.
If extra-articular disease is adequately controlled by an existing cDMARD but peripheral spondyloarthritis is not, consider adding another cDMARD. - Do not offer long-term (4 weeks or longer) treatment with antibiotics solely to manage reactive arthritis caused by a gastrointestinal or genitourinary infection after treating the initial reactive arthritis infection.
- Refer to specialist if person with axial spondyloarthritis presents with a suspected spinal fracture. Do not refer people with axial spondyloarthritis with spinal deformity to a complex spinal surgery service unless significantly affecting their quality of life and severe or progressing despite optimal non-surgical management.
- Advise that there may be a greater risk of skin cancer in people treated with TNF-alpha inhibitors.
- Discuss risk factors for cardiovascular comorbidities for all people with spondyloarthritis.
- Advise people with axial spondyloarthritis that they may be prone to fractures and consider regular osteoporosis assessments (every 2 years). Be aware that bone mineral density measures may be elevated on spinal dual-energy X-ray absorptiometry (DEXA) due to the presence of syndesmophytes and ligamentous calcification, whereas hip measurements may be more reliable.
NHS Somerset classify cDMARDS as Amber and biological DMARDS as Red, see Traffic light guidance.
NICE recommends a proton pump inhibitor (PPI) is co-prescribed when offering treatment with an oral NSAID.
- Use oral NSAIDs at the lowest effective dose for the shortest possible period of time.
NHS Somerset recommends people prescribed long term NSAIDs, antiplatelet or an anticoagulant should be considered for co-prescribing with a PPI to reduce GI bleed risk.
See NHS Somerset Formulary Gastric acid disorders and ulceration.
NICE recommends bone protection treatment should be considered in people taking high doses of glucocorticoids (prednisolone ≥7.5 mg daily or equivalent) for 3 months or longer. See Disorders of bone metabolism in formulary.
What should I consider when initiating NSAIDs? See Scenario: NSAIDs – prescribing issues (NICE CKS updated April 2020)
See NHS Somerset formulary Useful links for NHS Somerset sick day rules.
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Topical NSAIDs | - suitable for self-care | 5% gel: £2.50 (100g) | |
| 10% gel £5.79 (100g) | |||
| Piroxicam | 0.5% gel: £4.51 (112g) | ||
| Topical ketoprofen is no longer recommended following a Drug Safety Update warning about the risk of photosensitivity reactions after exposure to direct sunlight and UV lamps | |||
| Felbinac (Traxam®) and Diclofenac (Voltarol®) gels / foams are non formulary | |||
| Topical plant alkaloids | Capsaicin cream - Discontinued / non-formulary | ||
| NHS Somerset classify Qutenza® (Capsaicin cutaneous patch) as Red (specialist prescribing only) for the treatment of peripheral neuropathic pain in adults either alone or in combination with other medicinal products for the treatment of pain and not recommended for other indications as per Traffic light guidance. | |||
| Hexyl Nicotinate, Ethyl Nicotinate and Tetrahydrofurfuryl Salicylate (Transvasin®) Heat Rub Cream has been discontinued | Do not offer rubefacients for treating osteoarthritis | ||
| NSAIDs | We want to remind healthcare professionals that use of systemic (oral and injectable) NSAIDs such as ibuprofen, naproxen, and diclofenac is contraindicated in the last trimester of pregnancy (after 28 weeks of pregnancy). See MHRA (June 2023) for Non-steroidal anti-inflammatory drugs (NSAIDs): potential risks following prolonged use after 20 weeks of pregnancy. | ||
| Oral NSAIDs | - suitable for self-care | 200mg tablet: £3.08 (84) | First line |
| 400mg tablet: £2.98 (84) | |||
| 600mg tablet: £4.93 (84) | |||
| 100mg/5ml oral suspension sugar free: £2.93 (100ml) | |||
| Ibuprofen 200mg/5ml oral suspension sugar free: £3.49 (100ml) | |||
| Naproxen | 250mg tablet: £1.01 | Naproxen EC tablets are non formulary, evidence that EC reduces GI events is poor and they are three times the price of standard tablets. | |
| 500mg tablet: £1.32 | |||
| 250mg effervescent tablet: £52.72 (20) | |||
| Diclofenac is non formulary. Diclofenac: new contraindications and warnings. See MHRA (December 2014) for New recommendations after a Europe-wide review of cardiovascular safety. |
|||
| NHS Somerset classify Voltarol Pain-eze® Voltarol Rapid® as not recommended as per Traffic light guidance. | |||
| Oral COX-2 inhibitor NSAIDs | |||
| Meloxicam | 7.5mg tablet: £1.50 (30) | First-line | |
| 15mg tablet: £2.34 (30) | |||
| Celecoxib | 100mg capsule: £6.26 (60) | Etoricoxib & celecoxib are non formulary except Celecoxib for named-patient only when recommended by Consultant Rheumatologist when ibuprofen, naproxen and meloxicam are ineffective. Consider risk benefit compared to diclofenac. MHRA advised in October 2016 that the recommended dose of etoricoxib in RA and ankylosing spondylitis is reduced to 60mg once daily based on the balance of benefit and risks. |
|
| 200mg capsule: £5.61 (30) | |||
| Injectable corticosteroids | Methylprednisolone acetate as Depo-Medrone® | 40mg/1ml suspension for injection vial: £3.44 (1) | |
| 80mg/2ml suspension for injection vial: £6.18 (1) | |||
| 120mg/3ml suspension for injection vial: £8.96 (1) | |||
| Methylprednisolone acetate with lidocaine as Depo-Medrone with lidocaine® | 40mg/1ml / 10mg/1ml suspension for injection vial: £3.94 (1) | ||
| 80mg/2ml / 20mg/2ml suspension for injection vial: £7.06 (1) | |||
| Triamcinolone acetonide as Kenalog® | 40mg/1ml suspension for injection vial: £7.45 (5) | ||
| Triamcinolone hexacetonide as Esteve pharmaceuticals Ltd | 20mg/1ml suspension for injection ampoule: £120.00 (10) | ||
Aminosalicylates| Sulfasalazine | as Salazopyrin® 500mg tablet: £6.97 (112) |
| |
| 500mg suppository: £3.30 (10) | |||
| as Salazopyrin EN® | 500mg gastro resistant tablet: £8.43 (112) | ||
| Antineoplastic drugs, Antimetabolites | Methotrexate | 2.5mg tablets: £1.88 | For severe active rheumatoid arthritis if specialist initiates methotrexate under shared care. See Shared care protocol. Prescribe in multiple of 2.5mg tablets for safety reasons. 10mg are non formulary. |
| Methotrexate as Metoject® | 7.5mg/0.15ml injection: £12.87 (1) | SPS guide to methotrexate monitoring here Multiple DMARDs can be offered one after the other to achieve treatment targets. |
|
| 10mg/0.2ml injection: £13.26 (1) | |||
| 12.5mg/0.25ml injection £14.35 (1) | |||
| 15mg/0.3ml injection £14.41 (1) | |||
| 17.5mg/0.35ml injection £15.25 (1) | |||
| 20mg/0.4ml injection £15.56 (1) | |||
| 22.5mg/0.45ml injection £16.11 (1) | |||
| 25mg/0.5ml injection £16.64 (1) | |||
| 27.5mg/0.55ml injection £16.50 (1) | |||
| 30mg/0.6ml injection £16.56 (1) | |||
| as Nordimet® | 7.5mg/0.3ml solution for injection pre-filled disposable device: £13.37 (1) | ||
| 10mg/0.4ml solution for injection pre-filled disposable device: £13.77 (1) | |||
| 12.5mg/0.5ml solution for injection pre-filled disposable device: £14.85 (1) | |||
| 15mg/0.6ml solution for injection pre-filled disposable device: £14.92 (1) | |||
| 17.5mg/0.7ml solution for injection pre-filled disposable device: £15.75 (1) | |||
| 20mg/0.8ml solution for injection pre-filled disposable device: £16.06 (1) | |||
| 22.5mg/0.9ml solution for injection pre-filled disposable device: £16.61 (1) | |||
| 25mg/1ml solution for injection pre-filled disposable device: £16.64 (1) | |||
Figure 1 Visual summary on the management of osteoporosis
Figure 2 Rheumatoid arthritis: referral, diagnosis and investigations
Figure 3 Rheumatoid arthritis: management and monitoring