Related guidance:
Bisphosphonates for treating osteoporosis (TA464 August 2017, July 2019)
Hip fracture: management Clinical guideline (CG124 June 2011, updated January 2023)
NICE Decision aid for Bisphosphonates for treating osteoporosis (2019)
Early and locally advanced breast cancer: diagnosis and management NICE guideline (NG101 July 2018)
Bindex for investigating suspected osteoporosis Medtech innovation briefing (MIB106 May 2017)
- All patients recieving a course of corticosteriods for a disease flare should recieve an intake of 800-1000mg/day calcium and 800 units/day vitamin D (strong recommendation, very low-quality evidence). This can be achieved by administration of oral calcium and vitamin D supplements while on corticosteriods or vitamin D only if dietary calcium intake is adequate. Lifestyle modification advice including regular physical excercise and smoking cessation should be provided.
- Patients starting corticosteriods should be assessed for risk of osteoporosis. Those at high risk should be started on bisphosphonate therapy at the onset of corticosteroid therapy (strong recommendation, high-quality evidence), after ensuring adequate calcium intake and supplementing vitamin D.
FRAX® Fracture Risk Assessment Tool
MD+CALC Steroid Conversion Calculator
For Raloxifene, see Female sex hormone responsive conditions.
For Vitamin deficiency, see Blood and nutrition.
- Vitamin D supplementation alone or with calcium does not reduce fracture incidence among community-dwelling adults without known vitamin D deficiency, osteoporosis, or prior fracture.
- Bisphosphonates remain first line treatment option for people with osteoporosis or high risk of fractures.
- NHS Somerset have approved the use of Bisphosphonates (off-label) to address significant unmet need for people with high risk of fracture such as those with a diagnosis of osteopenia, who take aromatase inhibitors and high-dose oral or systemic glucocorticoids.
- NHS Somerset only recommend the use of Vitamin D3 and Calcium supplementation (see table below) alongside bone sparing treatment unless contraindicated. (with the exception of Bariatric patients as per SFT guidance).
- Calcium and vitamin D supplements may increase the risk of kidney stones. Calcium supplements can cause gastrointestinal upset, particularly constipation which can be a major issue in frail elderly people who are already prone to this problem. Frequency of acute admissions to hospital for abdominal problems doubled in those patients’ taking calcium.
- If people are concerned that they are not getting enough calcium in their diet, see Royal Osteoporosis Society calcium fact sheet with link to the calcium calculator.
Targeting risk assessment
- Consider in women aged ≥ 65 years and men aged ≥75 years.
- Consider in women aged <65 years and men aged <75 years in the presence of risk factors.
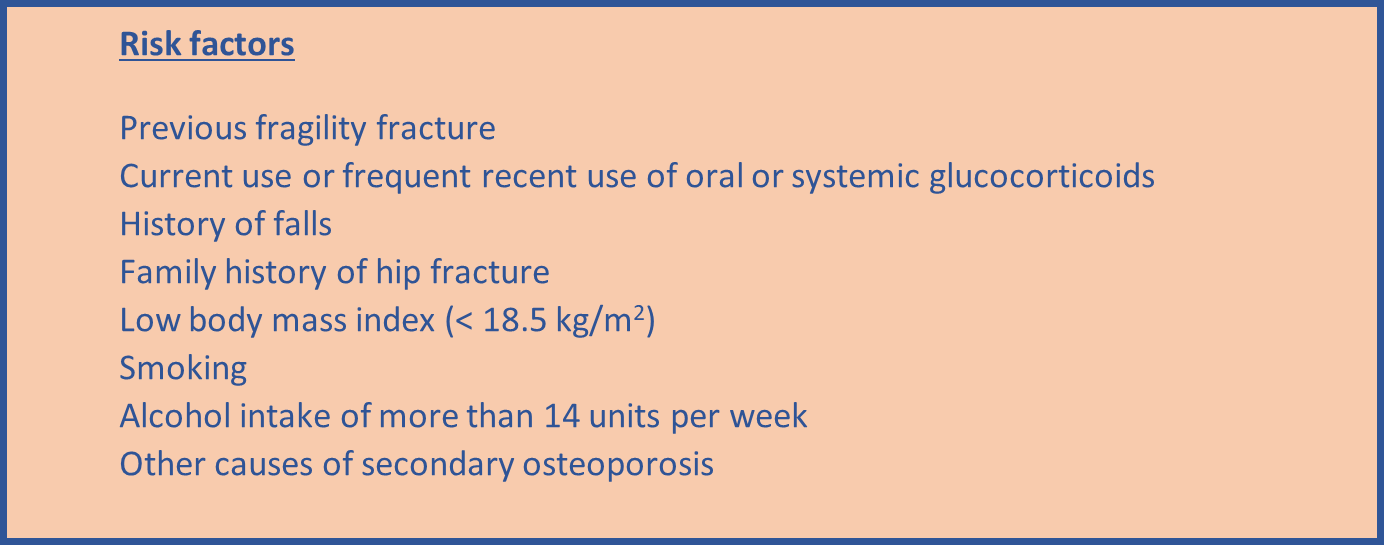
- Do not routinely assess fracture risk in people aged <50 years unless they have major risk factors for example, use oral or systemic glucocorticoids, untreated premature menopause or previous fragility fracture, because they are unlikely to be at high risk.
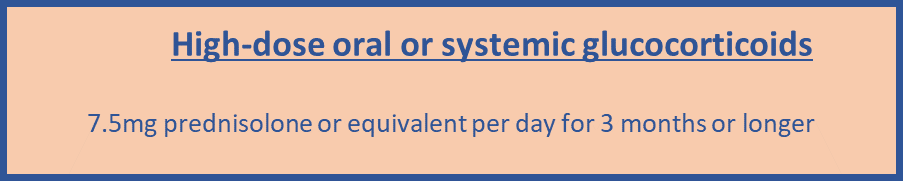
- Measure BMD to assess fracture risk in people aged under 40 years who have a major risk factor, such as history of multiple fragility fracture, major osteoporotic fracture, high-dose oral or systemic glucocorticoids.
- MD+CALC Steroid Conversion Calculator
Methods of risk assessment
- Estimate absolute risk when assessing risk of fracture for example, the predicted risk of major osteoporotic or hip fracture over 10 years, expressed as a percentage.
- Use either FRAX without a bone mineral density (BMD) value if a dual energy X-ray absorptiometry (DXA) scan has not previously been undertaken or QFracture within their allowed age ranges (FRAX age 40-90 and QFracture age 30-99) to estimate 10-year predicted absolute fracture risk when assessing risk of hip fracture alone and osteoporotic fracture (spine, forearm, hip or shoulder fracture).
- Above the upper age limits defined by the tools, consider people to be at high risk. Interpret the estimated absolute risk of fracture in people aged over 80 years with caution, because predicted 10-year fracture risk may underestimate their short-term fracture risk.
- Do not routinely measure BMD to assess fracture risk without prior assessment using FRAX or QFracture. Following risk assessment with FRAX or QFracture, consider measuring BMD with DXA in people whose fracture risk is in the region of an intervention threshold for a proposed treatment, and recalculate absolute risk using FRAX with the BMD value.
- Consider measuring BMD with DXA before starting treatments that may have a rapid adverse effect on bone density or example, sex hormone deprivation for treatment for breast or prostate cancer.
- Consider recalculating fracture risk in the future if the original calculated risk was in the region of the intervention threshold for a proposed treatment and only after a minimum of 2 years, or when there has been a change in the person’s risk factors.
- Take into account that risk assessment tools may underestimate fracture risk in certain circumstances for example if a person has a history of multiple fractures, has had previous vertebral fracture(s), has a high alcohol intake, is taking high-dose oral or systemic glucocorticoids or has other causes of secondary osteoporosis.
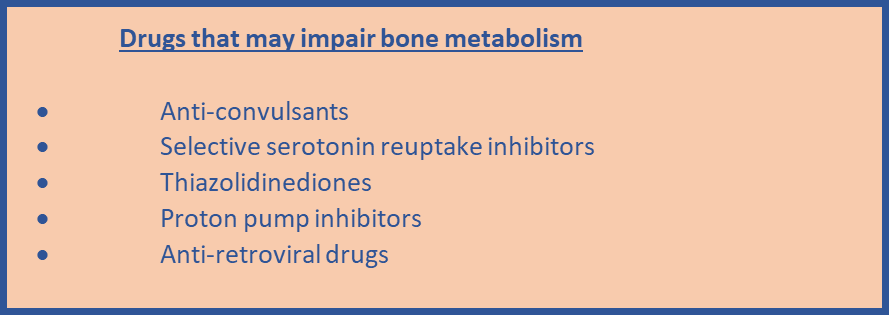
- Take into account that fracture risk can be affected by factors that may not be included in the risk tool, for example living in a care home or taking drugs that may impair bone metabolism.
- Because of concerns about rare but serious side-effects of long-term anti-resorptive therapy, many prescribe these drugs for a finite period of time, usually 3–5 years.
- Reassessment of fracture risk at the end of this treatment period is important, since some people remain at high risk of fracture and require continued treatment whereas others may benefit from a ‘drug holiday’ for 1 or more years. Neither FRAX nor QFracture has been examined in treated patients, and it is not known whether the ability of clinical risk factors with or without measurement of BMD to predict fracture risk is similar in untreated and treated patients.
- Bindex could be used after FRAX or QFracture assessment, in people with suspected osteoporosis. People with a high or intermediate risk classification based on FRAX would be scanned using Bindex. People whose Bindex scan gave a density index value that showed a high risk of osteoporosis would then be referred for osteoporosis treatment, without needing a DXA scan. Only people whose Bindex scan gave a density index value indicating an intermediate risk of osteoporosis would be referred for a DXA scan. See Bindex for investigating suspected osteoporosis.
- In postmenopausal women, the use of aromatase inhibitors increases bone turnover and induces bone loss at sites rich in trabecular bone at an average rate of 1–3% per year leading to an increase in fracture incidence compared to that seen during tamoxifen use. The bone loss is much more marked in young women with treatment-induced ovarian suppression followed by aromatase inhibitor therapy (average 7–8% per annum). Pre-treatment with tamoxifen for 2–5 years may reduce the clinical significance of the adverse bone effects associated with aromatase inhibitors, particularly if this leads to a shortening in the duration of exposure to an aromatase inhibitor. However, skeletal status should still be assessed at the commencement of aromatase inhibitor therapy. The rate of bone loss in women who experience a premature menopause before the age of 45 or are receiving ovarian suppression therapy is accelerated by the concomitant use of aromatase inhibitors. These patients are considered to be at high risk of clinically important bone loss and should have a baseline DXA assessment of BMD.
Vitamin D3 and Calcium formulations
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Calcium and vitamin D3 | Colecalciferol and calcium carbonate carbonate | For treatment with bone sparing agents except post bariatric procedures. | |
| as Evacal D3® | 400 unit and 1500mg chewable tablet: £2.75 (56) | Adult: Take one twice a day. Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Theical D3® | 880 unit and 1000mg chewable tablet: £2.95 (30) | Adult: Take one once a day. Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Adcal D3® | 200 unit and 750mg tablet: £3.54 (112) | Adult: Take two twice a day. Not suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| 400 unit and 1.5g chewable tablet: £4.38 (56) | Adult: Take one twice a day. (As above). |
||
| as Adcal D3 Dissolve® | 400 unit and 1.5g effervescent tablet: £5.99 (56) | Adult: Take one twice a day. Not suitable for peanut & soya allergy. Not suitable for Vegetarian. Not suitable for Vegan. Not suitable for Halal. Not suitable for Kosher. Suitable for lactose free. |
|
| as Calcichew D3 Forte® | 400 unit and 1.25g chewable tablet: £4.24 (60) | Adult: Take one twice a day. Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Calcichew D3 Once Daily® | 800 unit and 2.5g chewable tablet: £7.29 (30) | Adult: Take one once a day. Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
- Oral bisphosphonates are cost effective for people with at least a 1% fracture risk and intravenous bisphosphonates are cost effective for those with at least a 10% fracture risk.
- Some people will have difficulty adhering to the complex instructions for taking oral bisphosphonates, which will affect treatment benefit. For example, people with dementia, learning disabilities, those unable to remain upright for the specified time period, and people for whom oral bisphosphonates might be contraindicated such as those with oesophageal stricture, therefore an alternative route of administration may be considered.
See NICE patient decision aid What is osteoporosis and how can bisphosphonates help?
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments | |
|---|---|---|---|---|
| Bisphosphonates | Alendronic acid | 70mg tablet: £5.35 (4) | 1st line for primary and secondary prevention of of osteoporotic fragility fractures. The recommended dose is one 70mg tablet once a week. | People should be advised to swallow tablets whole with at least 200 ml of water on an empty stomach immediately after getting up in the morning. They should stay fully upright for at least 30 minutes or one hour after taking the tablet and before taking any food, drink or other medicine. They should also be advised to stop taking the tablets and to seek medical attention if they develop any symptoms of oesophageal irritation such as difficulty or pain upon swallowing, chest pain, or new or worsening heartburn. |
| as Binosto® | 70mg effervescent tablets sugar free: £11.60 (4) | |||
| 70mg/100ml oral solution unit dose sugar free: £32.45 (4) | ||||
| Risedronate sodium | 35mg tablet: £2.12 (4) | 2nd line for primary prevention and 1st line for secondary prevention of osteoporotic fragility fractures. The recommended dose is one 35mg tablet once a week. |
||
| Ibandronic acid | 150mg tablet: £1.66 (1) | 3rd line for primary and 2nd line for secondary prevention of osteoporosis. The recommended dose is one 150mg tablet once a month. |
||
| Sodium clodronate | 400mg capsule: £139.83 (120) | For prevention of osteoporotic fragility fractures, offer as adjuvant therapy to postmenopausal women with node-positive invasive breast cancer and consider as adjuvant therapy for postmenopausal women with node-negative invasive breast cancer and a high risk of recurrence. The recommended dose is 1.6g daily in 1-2 divided doses. |
||
| Zoledronic acid | 5mg/100ml solution for infusion vial: £253.58 (1) | 3rd line for secondary prevention of osteoporotic fragility fractures. For prevention of osteoporotic fragility fractures, offer as adjuvant therapy to postmenopausal women with node-positive invasive breast cancer and consider as adjuvant therapy for postmenopausal women with node-negative invasive breast cancer and a high risk of recurrence. Useful option for people who are unable to comply with the special instructions for oral bisphosphonates. The recommended dose is a single intravenous infusion of 5 mg administered once a year. | People must be appropriately hydrated prior to administration of zoledronic acid. This is especially important for ≥65 years and for patients receiving diuretic therapy. | |
| Discuss risks and benefits of treatment, particularly osteonecrosis of the jaw, atypical femoral fractures, osteonecrosis of the external auditory canal and atrial fibrillation. See MHRA Drug Safety Update (December 2014) for Bisphosphonates: use and safety. | ||||
| Osteonecrosis of the external auditory canal has been reported very rarely (fewer than 1 in 10 000 patients) with bisphosphonates, mainly in association with long-term therapy (2 years or longer). See MHRA Drug Safety Update (December 2015) for Bisphosphonates: very rare reports of osteonecrosis of the external auditory canal. | ||||
| Atypical femoral fractures reported rarely with bisphosphonate therapy, mainly in patients receiving long-term treatment for osteoporosis. See MHRA Drug Safety Update (December 2014) for Bisphosphonates: atypical femoral fractures. | ||||
| Clinical trial results suggest an increased risk of atrial fibrillation for zoledronic acid, pamidronic acid, and possibly for alendronic acid, although the balance-risk remains favourable for bisphosphonates. See MHRA Drug Safety Update (December 2014) for Bisphosphonates: atrial fibrillation. | ||||
| Monoclonal antibodies | Denosumab as Prolia® | Denosumab 60mg/1ml solution for injection pre-filled syringe: £183.00 (1) | For prevention of osteoporotic fracture in postmenopausal women and men (off label) at increased risk of fractures who are unable to comply with the special instructions for administering oral bisphosphonate, or have an intolerance of, or a contraindication to, those treatments. The recommended dose is 60 mg denosumab administered as a single subcutaneous injection once every 6 months into the thigh, abdomen or upper arm. |
|
| Xgeva® |
||||
| Denosumab is associated with a risk of osteonecrosis of the jaw (ONJ) and with a risk of hypocalcaemia. Check for ONJ risk factors before starting denosumab. A dental examination and appropriate preventive dentistry are now recommended for people with risk factors. Check calcium levels before each dose and within two weeks after the initial dose in people with risk factors for hypocalcaemia (e.g., severe renal impairment, creatinine clearance <30 ml/min) and if suspected symptoms of hypocalcaemia occur. Advise people to report symptoms of hypocalcaemia (e.g., muscle spasms, twitches, or cramps; numbness or tingling in the fingers, toes, or around the mouth). See MHRA Drug Safety Update (September 2014) for Denosumab: Minimising the risk of osteonecrosis of the jaw; monitoring for hypocalcaemia. | ||||
| Serious and life-threatening hypercalcaemia has been reported with denosumab 60mg in children and adolescents in clinical trials for osteogenesis imperfecta and during off-label use. Denosumab 60mg is authorised for use in adults with osteoporosis and other bone loss conditions – it should not be used in children and adolescents younger than 18 years. See MHRA Drug Safety Update (May 2022) for Denosumab 60mg: should not be used in patients under 18 years due to the risk of serious hypercalcaemia. | ||||
| An increased risk of multiple vertebral fractures has been reported in people within 18 months of stopping or delaying ongoing denosumab 60mg treatment for osteoporosis. People with a previous vertebral fracture may be at highest risk. Evaluate a person's individual factors for benefits and risks before initiating treatment with denosumab, particularly in people at increased risk of vertebral fractures for example those with previous vertebral fracture. People should not stop denosumab without specialist review. The optimal duration of denosumab treatment for osteoporosis has not been established; re-evaluate the need for continued treatment periodically based on the expected benefits and potential risks of denosumab on an individual basis, particularly after 5 or more years of use. Risks of long-term treatment with denosumab include rare cases of osteonecrosis of the jaw and atypical femoral fractures; osteonecrosis of the external auditory canal has also been reported in association with denosumab. See MHRA Drug Safety Update (August 2020) for Denosumab 60mg: increased risk of multiple vertebral fractures after stopping or delaying ongoing treatment. | ||||
| Parathyriod hormones and analogues | Teriparatide is classified by NHS Somerset as a Red drug as per Traffic light guidance. | |||