Related guidance:
Vitamin B12 deficiency in over 16s: diagnosis and management (NG239 March 2024)
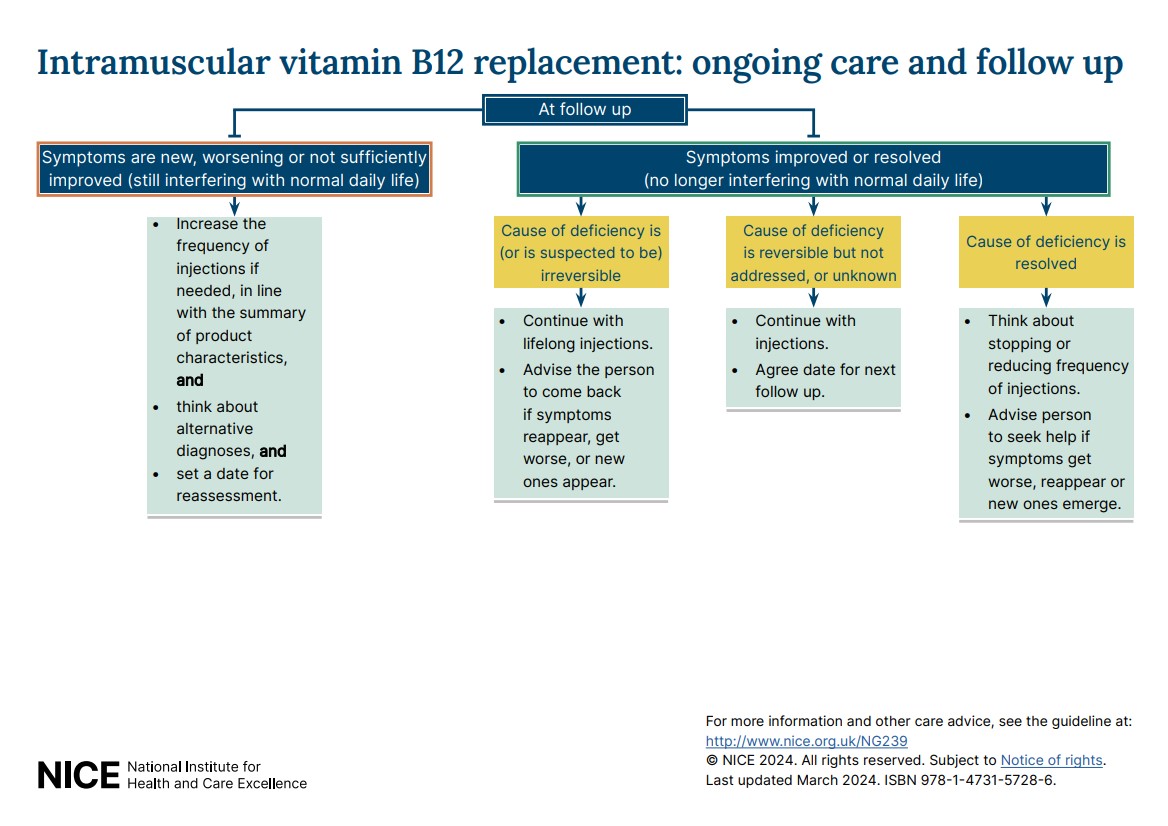
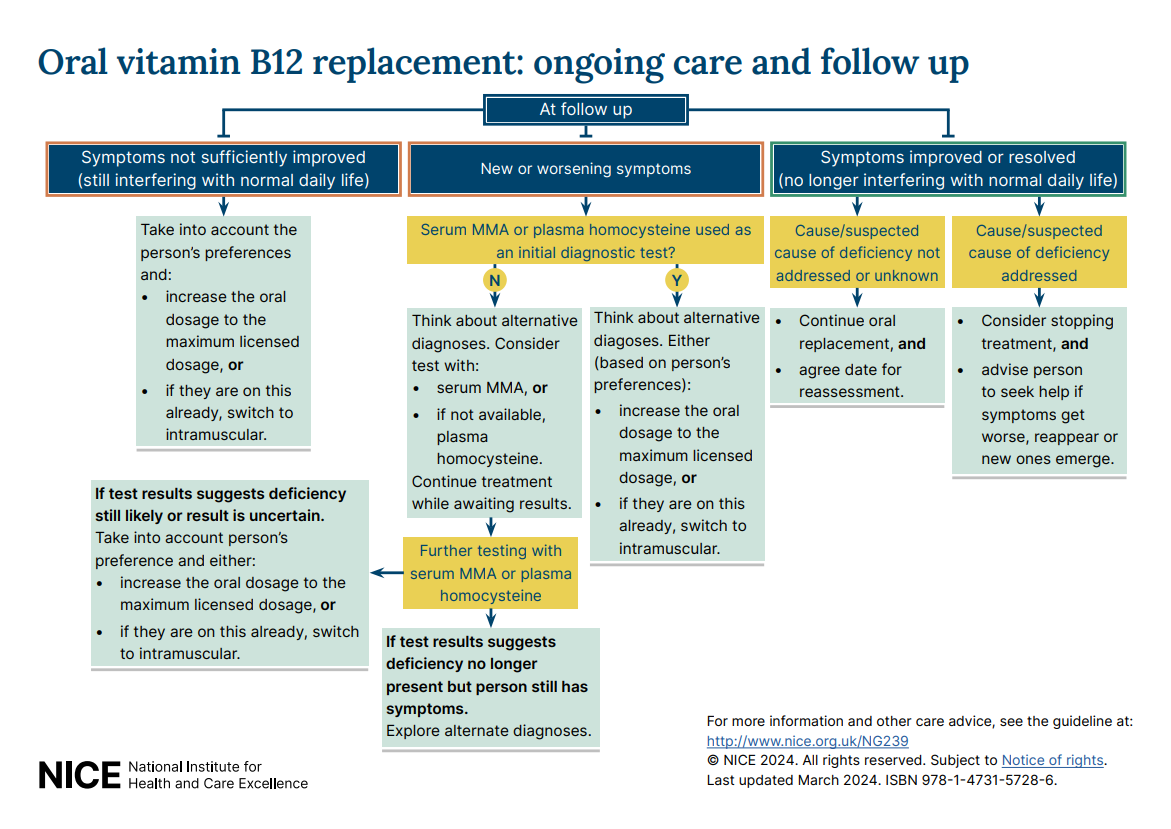
- Visual summary on ongoing care and follow-up options for oral and intramuscular vitamin B12 replacement.
Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline (NG49 July 2016)
Vitamins for dietary supplementation (including vitamin B12 for vegans and vegetaerians) should be purchased over the counter as per self-care, (except post bariatric surgery and renal dialysis).
Therapeutic Area Formulary Choices Cost for 28
(unless otherwise stated)Rationale for decision / comments
Vitamin B Vitamin B compound strong Tablet: £1.21 1 or 2 tablets, three times a day immediately before and during the first 10 days of feeding for people at high risk of developing refeeding problems.
Please do not prescribe Vitamin B compound tablets, these are not cost effective.
Thiamine (Vitamin B1) 100mg tablet: £4.97 (100) 100mg three times a day for those people immediately before and during the first 10 days of feeding for people at high risk of developing refeeding problems and people at high risk of developing, or with suspected Wernicke's encephalopathy.
The medicines used to treat vitamin B12 deficiency (hydroxocobalamin, cyanocobalamin) contain cobalt. There are case reports in the literature describing cobalt sensitivity-type reactions in patients being treated for vitamin B12 deficiency. See MHRA (December 2023) for Vitamin B12 (hydroxocobalamin, cyanocobalamin): advise patients with known cobalt allergy to be vigilant for sensitivity reactions.
Hydroxocobalamin (Vitamin B12) 1mg/1ml solution for injection ampoule: £10.78 (5) For prophylaxis of macrocytic anaemias associated with vitamin B12 deficiency.
1 mg by intramuscular injection every 2–3 months.
Cyanocobalamin (Vitamin B12)
1mg tablet: £9.99 (30) 1 mg once daily.
Vitamin K Phytomenadione (Vitamin K1) 10mg/1ml solution for injection ampoule: £10.49 (10)
2mg/0.2ml solution for injection ampoule: £10.49 (5)
See formulary section on oral anticoagulants for management of major bleed for people on warfarin.
2mg/0.2ml solution for injection ampoule: £10.49 (5)
Vitamin E Alpha-tocopherol equivalents (alpha-TE)
as Vitamin E Valupak®100 unit capsule: £0.87 (30) Secondary or tertiary centres may recommend the use of vitamin E for children or adults with advanced liver fibrosis. Offer to retest people after two years to assess whether treatment has been effective. If a persons ELF test score has risen (adults or children) stop Vitamin E.
400 unit capsule: £1.98 (30)
Alpha tocopheryl acetate (Tocopherol) 500mg/5ml oral suspension: £73.15 (100ml)
Multivitamin for adults with renal failure on dialysis
Renavit® Tablet: £13.79 (100) First line
Dialyvite® Unlicensed in UK – order supplies from USA Second line
Vitamin D3
Related guidance:
Vitamin D deficiency in adults (NICE CKS January 2022)
COVID-19 rapid guideline: (NICE December 2020)
Bisphosphonates for treating osteoporosis Technology appraisal guidance (NICE August 2017)
Over the counter medicines and homely remedies (Care Quality Commission May 2022)
Do not routinely test for vitamin D. (BMJ July 2022)
Osteoporosis: evidence for vitamin D and calcium in older people. (DTB August 2020)
Optimising the management of osteoporosis (Clinical Medicine 2020)
Management of osteoporosis and the prevention of fragility fractures (SIGN January 2021)
Clinician guideline for the prevention and treatment of osteoporosis. (NOGG September 2021)
- The entire population of the UK are at risk of having a low vitamin D status.
- Adults who are at higher risk of vitamin D deficiency include people aged 65 years and over who have low or no exposure to the sun, for example those who cover their skin; who are housebound or confined indoors for long periods, who have darker skin pigmentation, a malabsorption disorder, Coeliac disease, Cystic fibrosis, Crohn’s disease, following weight loss surgery, obesity, severe liver failure or end-stage chronic kidney disease, inherited enzyme disorders such as mutation of renal 25-hydroxyvitamin D 1 alpha-hydroxylase, taking certain drugs that reduce fat absorption (orlistat), antiepileptic drugs (especially carbamazepine, phenobarbital, and phenytoin), colestyramine, rifampicin, corticosteroids, thiazide diuretics, digoxin, calcium-channel blockers, antacids, highly active antiretroviral treatment (HAART) and are pregnant or breastfeeding. See NHS Somerset Vitamin D in pregnancy and breastfeeding.
- All adults should consider 400 IU (international units) (10 mcg) daily during autumn and winter and throughout the whole year if low or no exposure to the sun and minimal dietary intake. This includes those in institutions such as care homes, or who always cover their skin when outside, ethnic minority groups with dark skin, as may not get enough vitamin D from sunlight in the summer and who are pregnant or breast feeding. If you are unable to find a vitamin D supplement providing 10 micrograms (400 IU), products providing up to 25 micrograms (1000 IU) are suitable for everyone.
- Adults who are at higher risk of vitamin D deficiency may need to take a higher dose of vitamin D throughout the year. 800–2000 IU (20-50 mcg) daily and up to a maximum of 4000 IU (100mcg) daily for certain conditions such as malabsorption following specialist advice.
- Women and children who qualify for the Healthy Start scheme can get free supplements containing vitamin D. See NHS Somerset Infant Feeding for useful information and resources on Vitamin D and infant feeding.
- Normal usage of sunscreens does not generally prevent vitamin D synthesis.
- There is no evidence to support taking vitamin D supplements to specifically prevent or treat COVID-19.
- £136m could be saved by restricting prescribing for ‘minor’ conditions. Whilst Vitamin D maintenance therapy is recommended, there is no indication that this needs to be prescribed; vitamin D supplements can be bought cheaply and easily. Access to over the counter (OTC) medicines to self-care via Adult Social Care providers is an issue of equality. Policies should be in place to support people who wish to access OTC products in a timely manner.
- Routinely testing vitamin D levels in asymptomatic individuals is not recommended, based on a lack of evidence, except if osteomalacia is suspected and prior to osteoporosis treatment with a potent bone sparing agent (zoledronate, denosumab) or prior to Paget’s disease treatment with a bisphosphonate.
- Vitamin D supplementation alone or with calcium does not reduce fracture incidence among community-dwelling adults without known vitamin D deficiency, osteoporosis, or prior fracture.
- Vitamin D supplements only improve bone density and prevent fractures when marked deficiency is present and bone loss and fracture risk in older people are not related to calcium intake.
- NHS Somerset only recommend the use of Vitamin D3 and Calcium supplementation alongside bone sparing treatment unless contraindicated. (with the exception of Bariatric patients as per SFT guidance). Calcium and vitamin D supplements may increase the risk of kidney stones. Calcium supplements can cause gastrointestinal upset, particularly constipation which can be a major issue in frail elderly people who are already prone to this problem. Frequency of acute admissions to hospital for abdominal problems doubled in those patients’ taking calcium.
- See Disorders of bone metabolism for calcium and vitamin D formulations.
- Bisphosphonates remain first line treatment option for people with osteoporosis or high risk of fractures.
- NHS Somerset have approved the use of Bisphosphonates (off-label) to address significant unmet need for people with high risk of osteoporosis such as those with a diagnosis of osteopenia, who take aromatase inhibitors and long-term steroids (more than or equivalent to prednisolone 7.5 mg daily for 3 months or longer).
- Vitamin D3 is recommended as the vitamin D preparation of choice for treatment of vitamin D deficiency. Growing research suggests that vitamin D2 bioavailability may be significantly less when compared with vitamin D3 although further research is required.
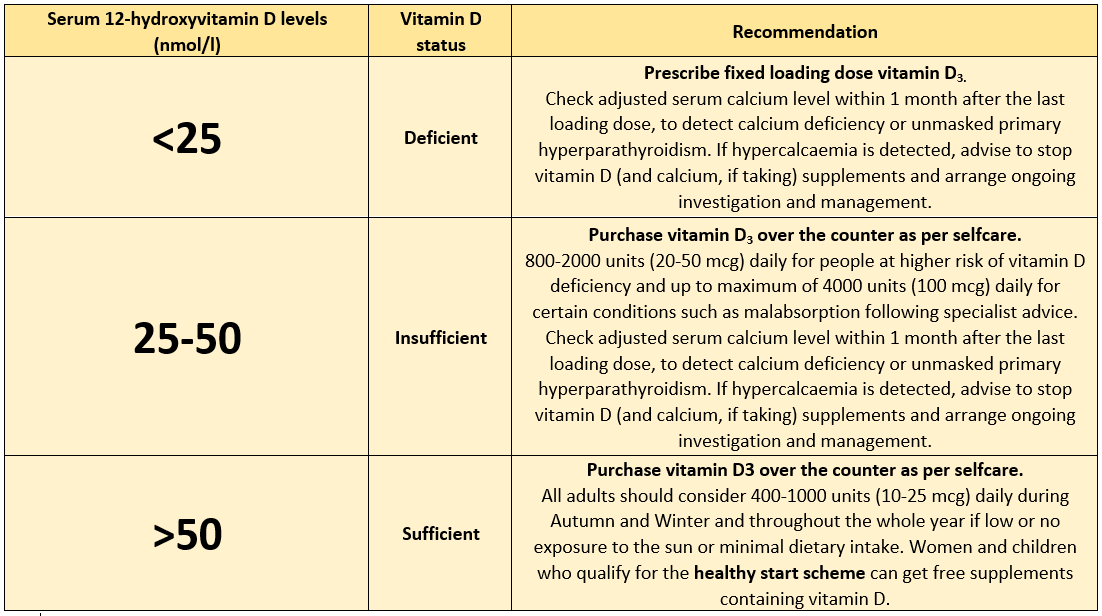
- Prescribe vitamin D3 as a fixed loading dose if rapid correction is needed to treat deficiency. Arrange referral and seek specialist advice for patients that are predisposed to hypercalcaemia, sarcoidosis, tuberculosis, metastatic bone disease, some lymphomas, or primary hyperparathyroidism, malabsorption, previous/active renal stone disease, severe kidney, or liver disease or pregnant.
- The Vitamin D3 loading regimen for treatment of deficiency should provide a total of approximately 300,000 IU of Vitamin D3, given either as separate weekly or daily doses over 6–10 weeks.
- Routine intermittent administration of large doses of vitamin D ≥60,000 IU is not advised, based on reports of an associated increased risk of fracture and falls.
- Check the adjusted serum calcium level within 1 month after the last loading dose, or after starting lower dose maintenance treatment with vitamin D, to detect calcium deficiency or unmasked primary hyperparathyroidism. If hypercalcaemia is detected, advise to stop vitamin D (and calcium, if taking) supplements and arrange ongoing investigation and management.
- Prescribe cost effectively and by brand to ensure a licensed product or preparation suitable for specific needs is dispensed.
The British Dietetic Association (BDA) Vitamin D Food Fact Sheet
NICE and PHE Vitamin D thresholds and dose recommendations
Vitamin D3 formulations
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Vitamin D3 | Colecalciferol | For vitamin D deficiency fixed loading doses. Adult: 50,000 units once weekly for 6 weeks (provides 300,000 units per course). 40,000 units once weekly for 7 weeks (provides 280,000 units per course). 4000 units once daily for 10 weeks (provides 280,000 units per course). |
|
| as ColeDose® | 4,000 unit tablet: £11.89 (70) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Suitable for Vegan. Suitable for Halal. Suitable for Kosher. Not suitable for lactose free. |
|
| 20,000 unit capsule: £1.44 (30) | Suitable for peanut & soya allergy. Not suitable for Vegetarian. Not suitable for Vegan. Not suitable for Halal. Not suitable for Kosher. Suitable for lactose free. |
||
| as Cubicole® | 20,000 unit capsule: £3.95 (30) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Plenacho D3® | 40,000 unit capsule: £10.50 (7) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as InVita D3® | 25,000 unit capsule: £3.95 (3) | Suitable for peanut & soya allergy. Not suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| 50,000 unit capsule: £4.95 (3) | |||
| 25,000 units/1ml oral solution unit dose ampoule sugar free: £4.45 (3) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Not suitable for Halal. Not suitable for Kosher. Suitable for lactose free. |
||
| 50,000 units/1ml oral solution unit dose ampoule sugar free: £6.25 (3) | |||
| as Pro D3® | 20,000 unit capsule: £19.99 (30) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Pro D3 vegan® | 20,000 unit capsule: £20.95 (30) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| as Thorens® | 10,000 units/ml oral solution sugar free: £5.85 (10ml) | Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Suitable for Halal. Suitable for Kosher. Suitable for lactose free. |
|
| 10,000 units/ml oral drop sugar free: £5.85 (10ml) | |||
| Calcifediol | as Domnisol® | 266microgram capsule: £1.99 (1), £5.97 (3) | For treatment of vitamin D deficiency in patients intolerant to Colecalciferol. Adults: 266 micrograms of calcifediol once a month. Higher doses may be necessary in some patients after analytical verification of the extent of the deficiency. In those cases, the maximum dose administered should not exceed one capsule per week. For patients who take bone sparing agents for fracture prevention intolerant to Colecalciferol. Adults: 266 micrograms of calcifediol once a month. Suitable for peanut & soya allergy. Suitable for Vegetarian. Not suitable for Vegan. Halal – contains small amount of alcohol. Suitable for Kosher. Suitable for lactose free. |