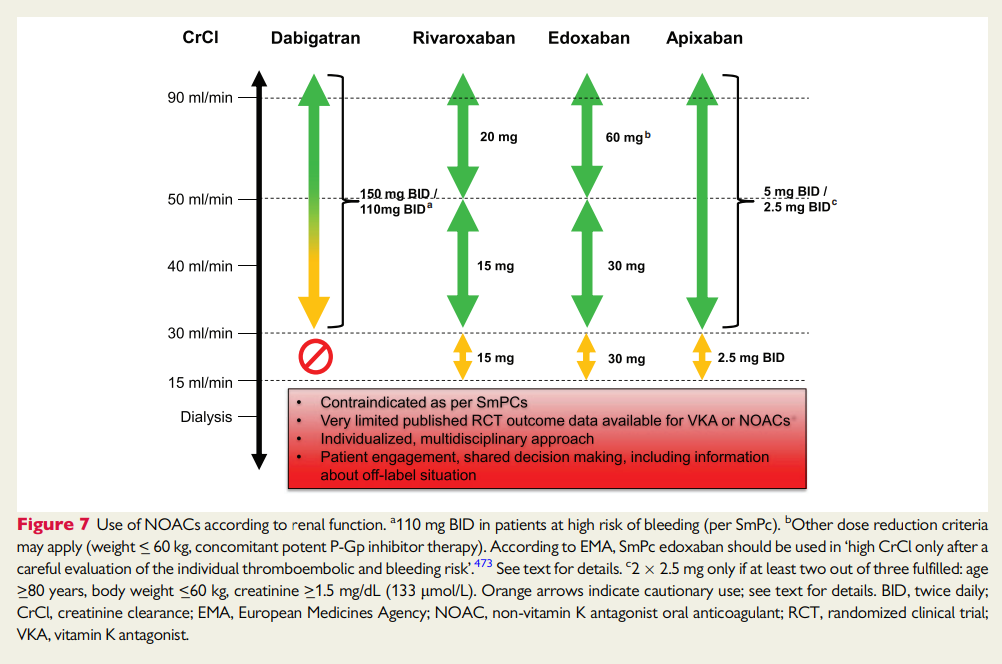
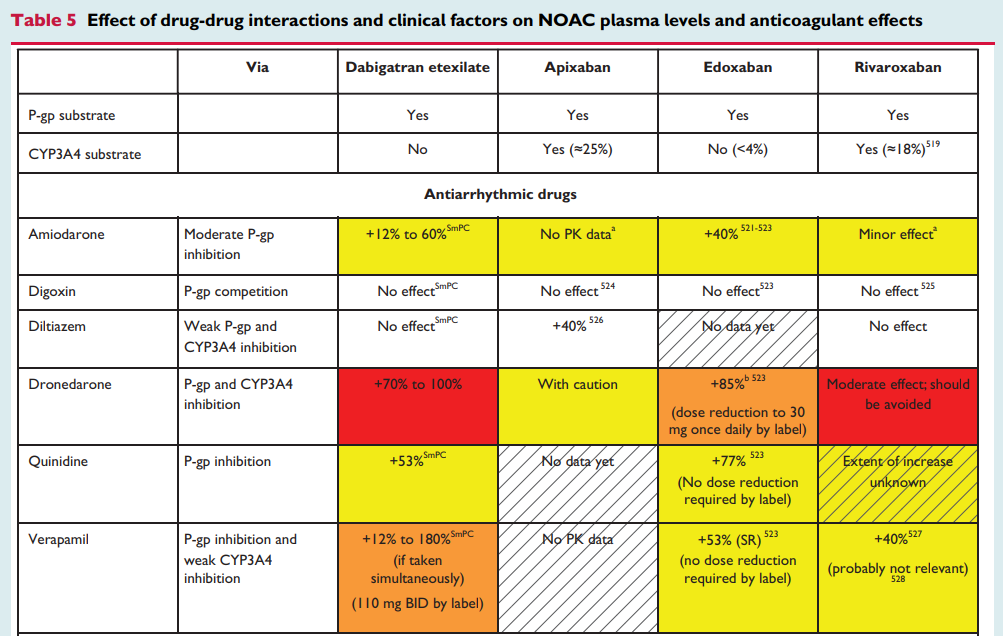
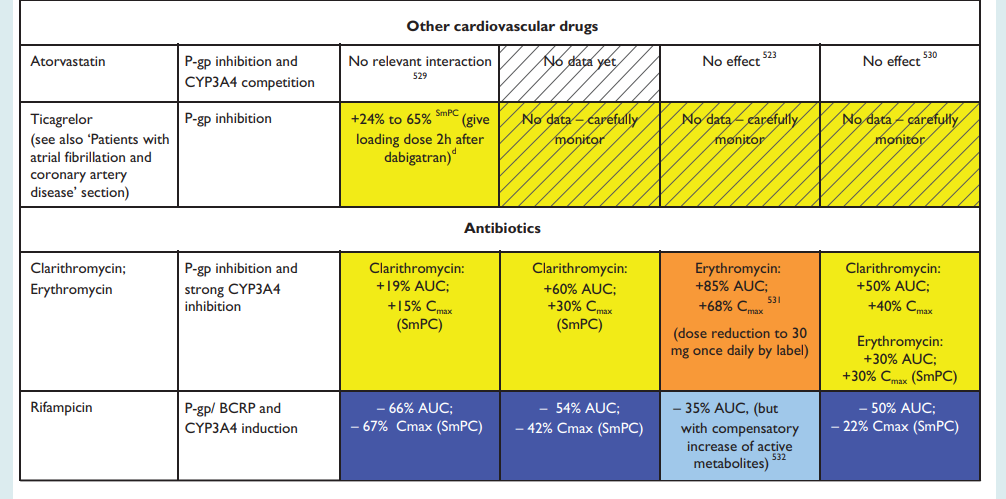
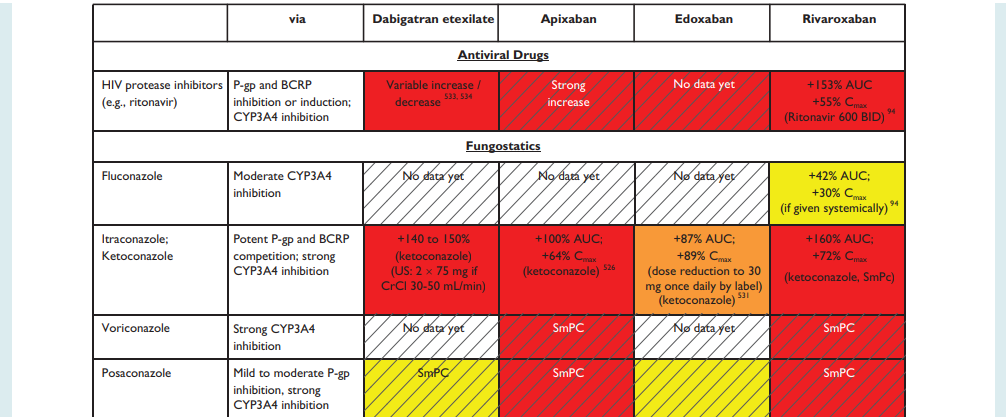
NHS Somerset recommends people prescribed long term NSAIDs, antiplatelet or an anticoagulant should be considered for co-prescribing with a PPI to reduce GI bleed risk.
For PPIs see NHS Somerset Formulary Gastric acid disorders and ulceration.
Related guidance:
For atrial fibrillation see NHS Somerset Formulary Arrhythmias.
Oral anticoagulants (NICE BNF Treatment summaries)
- The main adverse effect of all oral anticoagulants is haemorrhage. Checking the INR and omitting doses when appropriate is essential; if the anticoagulant is stopped but not reversed, the INR should be measured 2–3 days later to ensure that it is falling. The cause of an elevated INR should be investigated. The following recommendations (which take into account the recommendations of the British Society for Haematology Guidelines on Oral Anticoagulation with Warfarin—fourth edition. Br J Haematol 2011; 154: 311–324) are based on the result of the INR and whether there is major or minor bleeding; the recommendations apply to adults taking warfarin:
Major bleeding—stop warfarin sodium; give phytomenadione (vitamin K1) by slow intravenous injection; give dried prothrombin complex (factors II, VII, IX, and X); if dried prothrombin complex unavailable, fresh frozen plasma can be given but is less effective; recombinant factor VIIa is not recommended for emergency anticoagulation reversal.
INR >8.0, minor bleeding—stop warfarin sodium; give phytomenadione (vitamin K1) by slow intravenous injection; repeat dose of phytomenadione if INR still too high after 24 hours; restart warfarin sodium when INR <5.0
INR >8.0, no bleeding—stop warfarin sodium; give phytomenadione (vitamin K1) by mouth using the intravenous preparation orally [unlicensed use]; repeat dose of phytomenadione if INR still too high after 24 hours; restart warfarin when INR <5.0
INR 5.0–8.0, minor bleeding—stop warfarin sodium; give phytomenadione (vitamin K1) by slow intravenous injection; restart warfarin sodium when INR <5.0
INR 5.0–8.0, no bleeding—withhold 1 or 2 doses of warfarin sodium and reduce subsequent maintenance dose
Unexpected bleeding at therapeutic levels—always investigate possibility of underlying cause e.g. unsuspected renal or gastro-intestinal tract pathology.
For Phytomenadione (vitamin K1) see NHS Somerset Formulary Vitamin deficiency.
- Remain vigilant for signs and symptoms of bleeding complications during treatment with DOACs (apixaban, dabigatran, edoxaban, rivaroxaban), especially in patients with increased bleeding risks.
- Specific reversal agents are available for dabigatran (idarucizumab), and apixaban and rivaroxaban (andexanet alfa).
Actions that can make anticoagulant therapy safer (NPSA March 2007)
- This Patient Safety Alert advises healthcare organisations to take steps to manage the risks associated with the prescribing, dispensing and administering of anticoagulants.
- Anticoagulants are one of the classes of medicines most frequently identified as causing preventable harm and admission to hospital.
- Healthcare organisations should:
- Ensure staff are properly trained.
- Review and / or update written procedures and clinical protocols to ensure they reflect safe practice.
- Audit anticoagulant services using BSH/NPSA safety indicators as part of the annual medicines management audit programme.
- Ensure that patients prescribed anticoagulants receive appropriate information.
- Promote safe practice for prescribers and pharmacists to check that patients’ blood clotting (International Normalised Ratio, INR) is monitored regularly and that the INR level is safe before issuing or dispensing repeat prescriptions for oral anticoagulants.
- Promote safe practice for prescribers co-prescribing one or more clinically significant interacting medicines for patients already on oral anticoagulants: arrange for additional INR blood tests and inform the anticoagulant service that an interacting medicine has been prescribed.
- Ensure dental practitioners manage patients on anticoagulants according to evidence-based therapeutic guidelines.
- Amend local policies to standardise the range of anticoagulant products used, incorporating characteristics which promote safer use.
- Promote the use of written safe practice procedures for the administration of anticoagulants in social care settings.
The NPSA has developed some patient information, record books and alert cards for oral anticoagulant therapy, these should be issued to all patients.
Accessing resources for patients on high risk medicines (SPS)
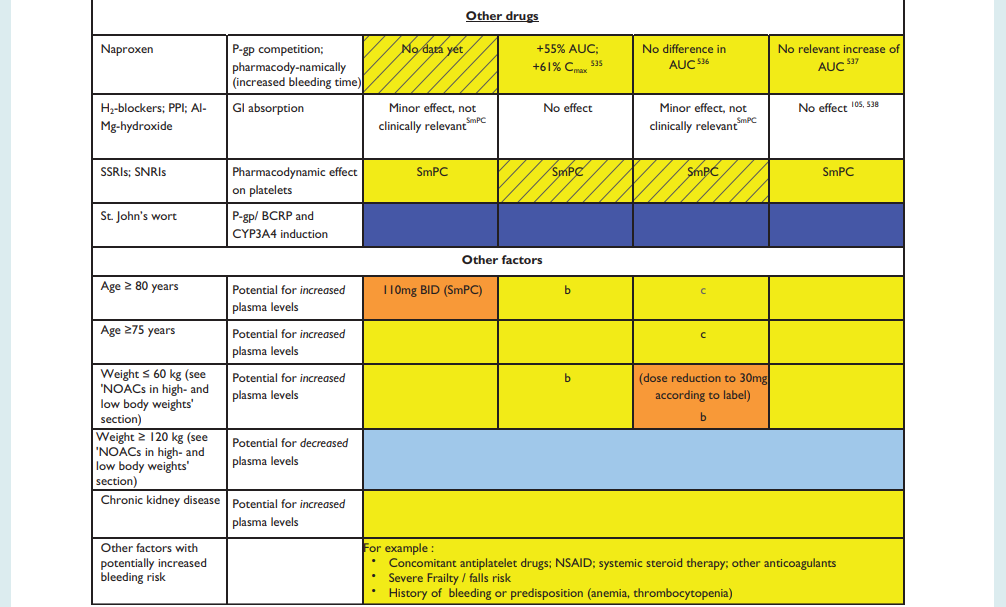
NHS Somerset Formulary for Interactions, see Appendix 1
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Antithrombotic drugs | |||
| Due to an increased bleeding risk, concomitant treatment with any other anticoagulants is contraindicated except under specific circumstances of switching anticoagulant therapy. Please follow manufacturers recommendations as per SPC. | |||
| Guidance has been published on monitoring of patients on warfarin and other anticoagulants during the COVID-19 pandemic. See MHRA (October 2020) for Warfarin and other anticoagulants: monitoring of patients during the COVID-19 pandemic. | |||
| Vitamin K antagonists | Management of patients on vitamin K antagonists should be in line with the local enhanced service. See Somerset LMC Anti-Coagulation Initiation, Stabilisation and Monitoring (October 2021). | ||
| Warfarin | 500mcg tablet: £1.63 | For prophylaxis of embolisation in atrial fibrillation: target INR = 2.5 For prophylaxis and treatment of DVT or PE: target INR = 2.5 For prophylaxis and treatment of recurrent DVT or PE: target INR = 3.5 For prophylaxis after insertion of mechanical aortic valve: target INR of 3 for mechanical mitral valve: target INR of 3.5 Caution in mild to moderate hepatic impairment and avoid in severe impairment. Antibiotics and diarrhoea may potentiate the effect of warfarin by reducing the absorption of vitamin K in the gut. Drugs co-administered may interact with warfarin and increase or decrease warfarin plasma concentrations and INR, potentially increasing the risk of bleeding or resulting in subtherapeutic effect. Warfarin dosage may need to be adjusted and the level of monitoring increased during this period and when starting and stopping these drugs. Glucosamine, cranberry juice and St John's Wort should be avoided. Certain foods such as liver, broccoli, Brussels sprouts and green leafy vegetables contain large amounts of vitamin K. Sudden changes in diet can potentially affect control of anticoagulation. Patients should be informed of the need to seek medical advice before undertaking any major changes in diet. |
|
| 1mg tablet: £0.89 | |||
| 3mg tablet: £1.05 | |||
| 5mg tablet: £1.88 | |||
| 1mg/ml oral suspension sugar free: £187.00 (150ml) | |||
| Thrombin inhibitors, direct | Risk of serious haemorrhage—clarified contraindications apply to all 3 medicines. See MHRA (December 2014) for New oral anticoagulants apixaban (Eliquis), dabigatran (Pradaxa) and rivaroxaban (Xarelto). | ||
| Contraindications clarified and reminder to monitor renal function. See MHRA (December 2014) for Dabigatran (Pradaxa): risk of serious haemorrhage. | |||
| Risk of thrombosis and haemorrhage in patients with prosthetic heart valve(s) requiring anti-coagulant treatment. See MHRA (December 2014) for Dabigatran (Pradaxa): contraindicated in patients with prosthetic heart valve(s) requiring anti-coagulant treatment. | |||
| A clinical trial has shown an increased risk of recurrent thrombotic events associated with rivaroxaban compared with warfarin, in patients with antiphospholipid syndrome and a history of thrombosis. Other direct-acting oral anticoagulants (DOACs) may be associated with a similarly increased risk. See MHRA (June 2019) for Direct-acting oral anticoagulants (DOACs): increased risk of recurrent thrombotic events in patients with antiphospholipid syndrome. | |||
| Risk minimisation materials are available to support the safe use of new paediatric formulations of rivaroxaban (Xarelto) and dabigatran etexilate (Pradaxa). In addition, we ask healthcare professionals to consult the current advice to ensure that all patients with renal impairment receive an appropriate dose of DOAC medicines. See MHRA (May 2023) for Direct-acting oral anticoagulants (DOACs): paediatric formulations; reminder of dose adjustments in patients with renal impairment. | |||
| Dabigatran etexilate as Pradaxa® | 75mg capsule: £51.00 (60) | For prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery* – RED DRUG Adult: 18–74 years 110 mg for 1 dose, to be taken 1–4 hours after surgery, followed by 220 mg once daily for 10 days for knee and 28-35 days for hip to be taken on the first day after surgery. Adult: 75 years and over, those with moderate renal impairment (CrCL 30-50 mL/min) and concomitant verapamil and amiodarone. 75 mg for 1 dose, to be taken 1–4 hours after surgery, followed by 150 mg once daily for 10 days for knee and 28-35 days for hip to be taken on the first day after surgery. For prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors. For the treatment of deep vein thrombosis and pulmonary embolism and prevention of recurrent deep vein thrombosis and pulmonary embolism in adults following treatment with a parenteral anticoagulant for at least 5 days Dose reduction for consideration for patients • 75-80 years • moderate renal impairment (CrCL 30-50 mL/min) • gastritis, esophagitis or gastroesophageal reflux • increased risk of bleeding Adult: 18–74 years 150 mg twice daily. Adult: 75–79 years 110–150 mg twice daily. Adult: 80 years and over or concomitant verapamil 110 mg twice daily. Short duration of therapy (at least 3 months) should be based on transient risk factors (e.g. recent surgery, trauma, immobilisation) and longer durations should be based on permanent risk factors or idiopathic DVT or PE. Missed dose: A forgotten dabigatran etexilate dose may still be taken up to 6 hours prior to the next scheduled dose. From 6 hours prior to the next scheduled dose on, the missed dose should be omitted. Concomitant use should be avoided with ketoconazole, dronedarone, itraconazole, cyclosporine, glecaprevir/pibrentasvir, rifampicin, St. John´s wort, carbamazepine, phenytoin and protease inhibitors such as ritonavir. Avoid if creatinine clearance less than 30 mL/minute. Avoid in severe hepatic impairment; consider avoiding in those with liver enzymes greater than 2 times the upper limit of normal. |
|
| 110mg capsule: £51.00 (60) | |||
| 150mg capsule: £51.00 (60) | |||
| *NHS Somerset classify Dabigatran as a Red drug (specialist prescribing only) for the prevention of venous thromboembolism after hip or knee replacement surgery as per Traffic light guidance. The full course is supplied by service provider post procedure. Patients must be closely monitored for signs of bleeding or anaemia. |
|||
| Factor Xa inhibitors | Apixaban | 2.5mg tablet: £4.92 (60) | For prevention of venous thromboembolic events in adult patients who have undergone elective hip or knee replacement surgery* – RED DRUG Adult: 2.5 mg twice daily for 10–14 days for knee and 32–38 days for hip, to be started 12–24 hours after surgery. First line for prophylaxis of stroke and systemic embolism in non-valvular atrial fibrillation and at least one risk factor (such as previous stroke or transient ischaemic attack, symptomatic heart failure, diabetes mellitus, hypertension, or age 75 years and over). Adult: 5 mg twice daily, alternatively 2.5 mg twice daily, reduced dose used in patients with at least two of the following characteristics: age 80 years and over, body-weight 60 kg or less, or serum creatinine 133 micromol/litre and over. First line for prophylaxis and treatment of deep-vein thrombosis pulmonary embolism. Treatment of deep-vein thrombosis and pulmonary embolism. Adult: initially 10 mg twice daily for 7 days, then maintenance 5 mg twice daily. Short duration of treatment (at least 3 months) should be based on transient risk factors (e.g., recent surgery, trauma, immobilisation). Prophylaxis of recurrent deep-vein thrombosis and recurrent pulmonary embolism. Adult: 2.5 mg twice daily, following completion of 6 months anticoagulant treatment. Missed dose: if a dose is missed, the patient should take apixaban immediately and then continue with twice daily intake as before. Avoid if creatinine clearance less than 15 mL/minute. Caution in mild to moderate hepatic impairment (or if hepatic transaminases greater than 2 times the upper limit of normal, or if bilirubin is equal or greater than 1.5 times the upper limit of normal); avoid in severe impairment or impairment associated with coagulopathy and clinically relevant bleeding risk. |
| 5mg tablet: £4.97 (56) | |||
| *NHS Somerset classify Apixaban as a Red drug (specialist prescribing only) for the prevention of venous thromboembolism after hip or knee replacement surgery as per Traffic light guidance. The full course is supplied by service provider post procedure. Patients must be closely monitored for signs of bleeding or anaemia. |
|||
| Edoxaban as Lixiana® | 15mg tablet: £17.50 (10) | Edoxaban 15 mg is not indicated as monotherapy. It is only indicated in the process of switching from edoxaban 30 mg to vitamin K antagonist. For patients currently on a 30 mg dose (for one or more of the following clinical factors: moderate to severe renal impairment (CrCl 15 – 50 mL/min), low body weight, or use with certain P-gp inhibitors), administer an edoxaban dose of 15 mg once daily together with an appropriate vitamin K antagonist dose. Patients should not take a loading dose of vitamin K antagonist in order to promptly achieve a stable INR between 2 and 3. It is recommended to take into account the maintenance dose of vitamin K antagonist if the patient was previously taking a vitamin K antagonist. Once an INR ≥ 2.0 is achieved, edoxaban should be discontinued. For prophylaxis of stroke and systemic embolism in non-valvular atrial fibrillation in patients with at least one risk factor (such as congestive heart failure, hypertension, aged 75 years and over, diabetes mellitus, previous stroke or transient ischaemic attack). For treatment and prophylaxis of deep-vein thrombosis and pulmonary embolism. Treatment should follow initial use of parenteral anticoagulant for at least 5 days. The duration of therapy for treatment of venous thromboembolism and prevention of recurrent VTE should be individualised after careful assessment of the treatment benefit against the risk for bleeding. Short duration of therapy (at least 3 months) should be based on transient risk factors (e.g. recent surgery, trauma, immobilisation) and longer durations should be based on permanent risk factors or idiopathic DVT or PE. Adult: (body-weight up to 61 kg) 30 mg once daily. Adult: (body-weight 61 kg and above) 60 mg once daily. Max. dose of 30 mg once daily with concurrent ciclosporin, dronedarone, erythromycin, or ketoconazole. Use a dose of 30 mg once daily if creatinine clearance 15–50 mL/minute. Missed dose: if a dose of edoxaban is missed, the dose should be taken immediately and then be continued the following day with the once-daily intake as recommended. The patient should not take double the prescribed dose on the same day to make up for a missed dose. Avoid if creatinine clearance less than 15 mL/minute. Caution in mild to moderate hepatic impairment, or if liver transaminases greater than 2 times the upper limit of normal, or total bilirubin 1.5 times the upper limit of normal or greater; avoid in severe impairment or hepatic disease associated with coagulopathy and clinically relevant bleeding risk. |
|
| 30mg tablet: £49.00 | |||
| 60mg tablet: £49.00 | |||
| A clinical trial has shown an increased risk of recurrent thrombotic events associated with rivaroxaban compared with warfarin, in patients with antiphospholipid syndrome and a history of thrombosis. Other direct-acting oral anticoagulants (DOACs) may be associated with a similarly increased risk. See MHRA (June 2019) for Direct-acting oral anticoagulants (DOACs): increased risk of recurrent thrombotic events in patients with antiphospholipid syndrome. | |||
| Rivaroxaban as Xarelto® | 10mg tablet: £54.00 (30) | For prevention of venous thromboembolism in adult patients undergoing elective hip or knee replacement surgery* – RED DRUG Adult: 10 mg once daily for 2 weeks following knee and for 5 weeks following hip replacement, to be started 6–10 hours after surgery. For some patients with thrombophlebitis secondary care may recommend a course of rivaroxaban 10mg (off-label). For prophylaxis of stroke and systemic embolism in patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack. Adult: 20 mg once daily. In patients with moderate (creatinine clearance 30 - 49 ml/min) or severe (creatinine clearance 15-29 ml/min) renal impairment the recommended dose is 15 mg once daily. If a dose is missed the patient should take Xarelto immediately and continue on the following day with the once daily intake as recommended. The dose should not be doubled within the same day to make up for a missed dose. For prophylaxis and treatment of deep-vein thrombosis pulmonary embolism. Adult: initially 15 mg twice daily for 21 days, then maintenance 20 mg once daily. In patients with moderate (creatinine clearance 30 - 49 ml/min) or severe (creatinine clearance 15 - 29 ml/min) renal impairment a reduction of the dose to 15 mg once daily should be considered if the patient's assessed risk for bleeding outweighs the risk for recurrent DVT and PE. If a dose is missed during the 15 mg twice daily treatment phase (day 1 - 21), the patient should take Xarelto immediately to ensure intake of 30 mg Xarelto per day. In this case two 15 mg tablets may be taken at once. The patient should continue with the regular 15 mg twice daily intake as recommended on the following day. Short duration of therapy (at least 3 months) should be considered in patients with DVT or PE provoked by major transient risk factors (i.e. recent major surgery or trauma). Longer duration of therapy should be considered in patients with provoked DVT or PE not related to major transient risk factors, unprovoked DVT or PE, or a history of recurrent DVT or PE. When extended prevention of recurrent DVT and PE is indicated (following completion of at least 6 months therapy for DVT or PE), the recommended dose is 10 mg once daily. In patients in whom the risk of recurrent DVT or PE is considered high, such as those with complicated comorbidities, or who have developed recurrent DVT or PE on extended prevention with Xarelto 10 mg once daily, a dose of Xarelto 20 mg once daily should be considered. For patients who are unable to swallow whole tablets, Xarelto tablet may be crushed and mixed with water or apple puree immediately prior to use and administered orally. After the administration of crushed Xarelto 15 mg or 20 mg film-coated tablets, the dose should be immediately followed by food. The crushed tablet may also be given through gastric tubes. Avoid if creatinine clearance less than 15 mL/minute. Avoid in hepatic disease with coagulopathy and clinically-relevant bleeding risk including patients with moderate to severe cirrhosis. Should avoid ritonavir, ketoconazole, itraconazole, voriconazole, posaconazole, HIV protease inhibitors and dronedarone. |
|
| 15mg tablet: £50.40 | |||
| 20mg tablet: £50.40 | |||
| 1mg/ml oral suspension sugar free: £18.00 (250ml) | |||
| *NHS Somerset classify Rivaroxaban as a Red drug (specialist prescribing only) for the prevention of venous thromboembolism after hip or knee replacement surgery as per Traffic light guidance. The full course is supplied by service provider post procedure. Patients must be closely monitored for signs of bleeding or anaemia. |
|||
| Antidotes and chelators | NHS Somerset classify Idarucizumab as a Red drug (specialist prescribing only) for the rapid reversal of dabigatran for emergency procedures or in life-threatening or uncontrolled bleeding as per Traffic light guidance. | ||
| NHS Somerset classify Andexanet as a Red drug (specialist prescribing only) for the reversal of apixaban or rivaroxaban in life-threatening or uncontrolled bleeding as per Traffic light guidance. | |||