1.6.1 Dyspepsia
1.6.2 Gastric and duodenal ulceration
1.6.3 Gastro-oesophageal reflux disease
1.6.4 Helicobacter pylori infection
Related guidance:
NICE Decision aid for long term heart burn (September 2014)
Scenario: NSAIDs – prescribing issues (NICE CKS updated April 2020)
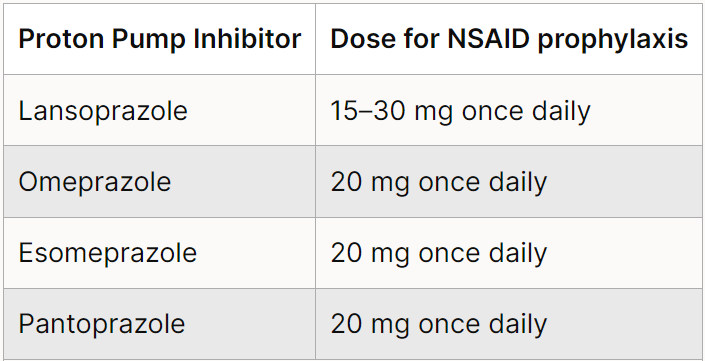
NHS Somerset recommends people prescribed long term NSAIDs, antiplatelet or an anticoagulant should be considered for co-prescribing with a PPI to reduce GI bleed risk.
See below licensed doses of proton pump inhibitors used for gastroprotection.
Dyspepsia
- Community pharmacists should offer initial and ongoing help for people with symptoms of dyspepsia, including advice about lifestyle changes, over-the-counter medication, prescribed drugs, when to consult a GP, recording adverse reactions to treatment and medication review.
- Offer lifestyle advice on healthy eating, weight reduction and smoking cessation and advise people to avoid known precipitants they associate with their dyspepsia where possible. These include smoking, alcohol, coffee, chocolate, fatty foods and being overweight. Raising the head of the bed and having a main meal well before going to bed may help.
- Provide people with access to educational materials to support the care they receive. See Guts UK for patient information on Indigestion.
- Recognise that psychological therapies, such as cognitive behavioural therapy and psychotherapy, may reduce dyspeptic symptoms in the short term in individual people.
People presenting with dyspepsia together with significant acute gastrointestinal bleeding, require immediate referral (on the same day) to a specialist.
- Review medications for possible causes of dyspepsia (calcium antagonists, nitrates, theophyllines, bisphosphonates, corticosteroids and non-steroidal anti-inflammatory drugs [NSAIDs]). In people needing referral, suspend NSAID use. Think about the possibility of cardiac or biliary disease as part of the differential diagnosis. If people have had a previous endoscopy and do not have any new alarm signs, consider continuing management according to previous endoscopic findings.
- Dyspepsia is defined broadly to include people with recurrent epigastric pain, heartburn or acid regurgitation, with or without bloating, nausea or vomiting.
- For people with uninvestigated dyspepsia, leave a 2-week washout period after proton pump inhibitor (PPI) use before testing for Helicobacter pylori (H pylori) with a breath test or a stool antigen test.
- Offer empirical full-dose PPI therapy (see table 1) for 4 weeks and H pylori ‘test and treat’ to people with dyspepsia. If symptoms return after initial care strategies, step down PPI therapy to the lowest dose needed to control symptoms. Discuss using the treatment on an ‘as-needed’ basis with people to manage their own symptoms. Offer H2 receptor antagonist (H2RA) therapy if there is an inadequate response to a PPI.
- Offer people who need long-term management of dyspepsia symptoms an annual review of their condition, and encourage them to try stepping down or stopping treatment (unless there is an underlying condition or comedication that needs continuing treatment). Advise people that it may be appropriate for them to return to self-treatment with antacid and/or alginate therapy (purchased over-the-counter and taken as needed).
Functional dyspepsia
- Manage endoscopically determined functional dyspepsia using initial treatment for H pylori if present, followed by symptomatic management and periodic monitoring.
- Offer eradication therapy to people testing positive for H pylori. Do not routinely offer re-testing after eradication.
- If H pylori has been excluded and symptoms persist, offer either a low dose PPI (see table 1) or an H2RA for 4 weeks.
- If symptoms continue or recur after initial treatment, offer a PPI or H2RA to be taken at the lowest dose possible to control symptoms.
- Discuss using PPI treatment on an ‘as-needed’ basis with people to manage their own symptoms.
- Avoid long-term, frequent dose, continuous antacid therapy (it only relieves symptoms in the short term rather than preventing them).
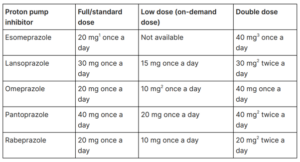
Table 1 Dosage information on PPIs (NICE CG184 September 2014, updated October 2019)
Peptic ulcer disease
- Offer H pylori eradication therapy to people who have tested positive for H pylori and who have peptic ulcer disease.
- For people using NSAIDs with diagnosed peptic ulcer, stop the use of NSAIDs where possible. Offer full-dose PPI (see table 1) or H2RA therapy for 8 weeks and, if H pylori is present, subsequently offer eradication therapy.
- Offer people with gastric ulcer and H pylori repeat endoscopy 6 to 8 weeks after beginning treatment, depending on the size of the lesion.
- Offer people with peptic ulcer (gastric or duodenal) and H pylori retesting for H pylori 6 to 8 weeks after beginning treatment, depending on the size of the lesion.
- Offer full-dose PPI (see table 1) or H2RA therapy for 4 to 8 weeks to people who have tested negative for H pylori who are not taking NSAIDs.
- For people continuing to take NSAIDs after a peptic ulcer has healed, discuss the potential harm from NSAID treatment. Review the need for NSAID use regularly (at least every 6 months) and offer a trial of use on a limited, ‘as-needed’ basis. Consider reducing the dose, substituting an NSAID with paracetamol, or using an alternative analgesic or low-dose ibuprofen (1.2 g daily).
- In people at high risk (previous ulceration) and for whom NSAID continuation is necessary, consider a COX-2 selective NSAID instead of a standard NSAID. In either case, prescribe with a PPI.
- In people with an unhealed ulcer, exclude non-adherence, malignancy, failure to detect H pylori, inadvertent NSAID use, other ulcer-inducing medication and rare causes such as Zollinger–Ellison syndrome or Crohn’s disease.
- If symptoms recur after initial treatment, offer a PPI to be taken at the lowest dose possible to control symptoms.
- Discuss using the treatment on an ‘as-needed’ basis with people to manage their own symptoms.
- Offer H2RA therapy if there is an inadequate response to a PPI.
Gastro-oesophageal reflux disease (GORD)
- Manage uninvestigated ‘reflux-like’ symptoms as uninvestigated dyspepsia.
- Offer people with GORD a full-dose PPI (see table 1) for 4 or 8 weeks. If symptoms recur after initial treatment, offer a PPI at the lowest dose possible to control symptoms. Discuss with people how they can manage their own symptoms by using the treatment when they need it. Offer H2RA therapy if there is an inadequate response to a PPI.
- See Guts UK for patient information on Heartburn and Acid Reflux.
- People who have had dilatation of an oesophageal stricture should remain on long-term full-dose PPI (see table 1) therapy.
- Offer people a full-dose PPI (see table 2) for 8 weeks to heal severe oesophagitis, taking into account the person’s preference and clinical circumstances (underlying health conditions and possible interactions with other drugs).
- If initial treatment for healing severe oesophagitis fails, consider a high dose of the initial PPI, switching to another full-dose PPI or switching to another high-dose PPI, taking into account the person’s preference and clinical circumstances (tolerability of the initial PPI, underlying health conditions and possible interactions with other drugs).
- Offer a full-dose PPI long-term as maintenance treatment for people with severe oesophagitis.
- If the person’s severe oesophagitis fails to respond to maintenance treatment, carry out a clinical review. Consider switching to another PPI at full dose or high dose and/or seeking specialist advice. Do not routinely offer endoscopy to diagnose Barrett’s oesophagus, but consider it if the person has GORD. Discuss the person’s preferences and their individual risk factors (long duration of symptoms, increased frequency of symptoms, previous oesophagitis, previous hiatus hernia, oesophageal stricture or oesophageal ulcers, or male gender).
- Consider laparoscopic fundoplication for people who have a confirmed diagnosis of acid reflux and adequate symptom control with acid suppression therapy, but who do not wish to continue with this therapy long term and a confirmed diagnosis of acid reflux and symptoms that are responding to a PPI, but who cannot tolerate acid suppression therapy.
- Refer to specialist people of any age with gastro-oesophageal symptoms that are non-responsive to treatment or unexplained, suspected GORD who are thinking about surgery and with H pylori that has not responded to second-line eradication therapy.
- Consider surveillance to check progression to cancer for people who have a diagnosis of Barrett’s oesophagus (confirmed by endoscopy and histopathology), taking into account the presence of dysplasia, the person’s individual preference and the person’s risk factors (male gender, older age and the length of the Barrett’s oesophagus segment). Emphasise that the harms of endoscopic surveillance may outweigh the benefits in people who are at low risk of progression to cancer (stable non-dysplastic Barrett’s oesophagus).
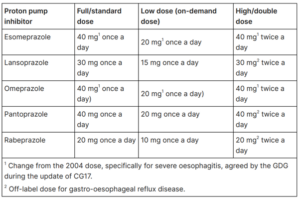
Table 2 Dosage information on PPIs (NICE CG184 September 2014, updated October 2019)
Helicobacter pylori infection
- Test for H pylori using a carbon-13 urea breath test or a stool antigen test, or laboratory-based serology where its performance has been locally validated.
- Perform re-testing for H pylori using a carbon-13 urea breath test.
- Do not use office-based serological tests for H pylori because of their inadequate performance.
- See Guts UK for patient information on H pylori.
- For eradication see NHS Somerset Managing common infections guidance for Primary Care.
Dyspepsia – unidentified cause (NICE CKS updated July 2022)
- PPIs or H2RAs should not be prescribed to people with alarm symptoms before endoscopy, as may mask the symptoms of upper gastrointestinal malignancy. If the person is already taking a PPI and subsequently needs an endoscopy, the PPI should be stopped at least 2 weeks before the procedure.
- PPIs should be prescribed with caution to people at risk of osteoporosis — the person should be given bone-sparing therapy with calcium and vitamin D supplement. See Disorders of bone metabolism in formulary. Those at risk of hypomagnesaemia — if possible, magnesium levels should be checked before starting PPI therapy and intermittently during long-term treatment, for example if the person is prescribed drugs that can cause hypomagnesaemia, such as digoxin and diuretics.
- Other conditions which may also present with dyspepsia symptoms include: Upper gastrointestinal malignancy, Gallbladder or hepatobiliary disease, Pancreatic disease, Cardiac disease, Gastroenteritis, Coeliac disease, Crohn’s disease, Irritable bowel syndrome, Small intestine bacterial overgrowth — may also present with weight loss, chronic diarrhoea, and malabsorption and Abdominal aortic aneurysm (rare).
Use of either omeprazole or esomeprazole with clopidogrel should be discouraged. See Clopidogrel and PPIs: interaction—updated advice (MHRA December 2014)
- Prescribe Pantoprazole tablets or Lansoprazole capsules or Rabeprazole tablets (second- and third-line options if Pantoprazole is unsuitable).
PPIs deprescribing algorithm (Dorset CCG July 2016)
Heartburn and Dyspepsia: treatment during pregnancy (SPS January 2022)