Related guidance:
Tirzepatide for treating type 2 diabetes Technology appraisal guidance (TA924 25 October 2023)
Information for drivers with diabetes treated by non insulin medication, diet, or both (DVLA)
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Biguanides | Metformin Immediate-release | 500mg tablets: £0.95(28) 850mg tablets: £2.61 (56) | Offer standard-release metformin as the initial drug treatment for adults with type 2 diabetes Gradually increase the dose of standard-release metformin over several weeks to minimise the risk of gastrointestinal side effects in adults with type 2 diabetes e.g. 500mg daily and gradually titrated to 2g per day (or 3g under specialist supervision). -Continued in patients with Type 2 DM who require Insulin, as Metformin reduces insulin requirements. - Use with caution in those at risk of a sudden deterioration in kidney function Metformin has a risk of lactic acidosis in patients with reduced renal function and is contraindicated below eGFR of 30ml/min. The European Medicines Agency (EMA) has concluded that metformin-containing medicines can now be used in patients with moderately reduced kidney function (GFR [glomerular filtration rate]=30–59 ml/min) for the treatment of type 2 diabetes |
| Metformin 500mg oral powder sachets sugar free (Morningside Healthcare Ltd) | £6.30 (30) | First line for patients unable to swallow solid dosage forms | |
| Metformin 500mg/5ml oral solution sugar free | £37.45 (150ml) | Alternative for patients unable to swallow solid dosage forms | |
| First-line: Modified release as generic | 500mg S/R tablets: £1.80 (56) 750mg S/R tablets: £2.87 (56) Tariff is £6.40 1000mg S/R tablets:£3.82 (56) 750mg M/R tablets: £2.88 (56) Tariff is £6.40 1000mg S/R tablets: £3.83 (56) | If an adult with type 2 diabetes experiences gastrointestinal side effects with standard-release metformin, consider a trial of modified-release metformin. |
|
| Sodium-glucose cotransporter-2 (SGLT-2) inhibitor GLIFLOZINS | Type 2 diabetes treatment only-do not use in T1 diabetes due to risk of DKA Due to recent studies and updated NICE guidance, SGLT-2 inhibitors (gliflozins) are much favoured as they have been shown to improve outcomes in cardiovascular disease, heart failure and chronic kidney disease. SGLT-2 inhibitors are preferred over DPP-4 inhibitors in most patients. Frail elderly may benefit most from the latter. For advice on who would be suitable for SGLT-2 inhibitors visit: ABCD guide to SGLT-2 inhibitors in Type 2 diabetes Patients treated with gliflozins must keep well hydrated and check for signs of DKA, preferably given guidance on how to check ketone levels |
||
| SURGICAL TREATMENT. SGLT2 inhibitor treatment should be interrupted in patients who are hospitalised for major surgical procedures or acute serious medical illnesses and ketone levels measured, preferably in blood rather than urine. Treatment may be restarted when the ketone values are normal and the patient's condition has stabilised. MHRA alert March 2020 EMA could not find evidence to guide on when to stop or restart SGLT-2s but FDA recommendations are: Canagliflozin,dapagliflozin,and empagliflozin should each be temporarily discontinued at least 3 days prior to scheduled surgery. Ertugliflozin should be temporarily discontinued at least 4 days prior to scheduled surgery. Blood glucose levels should be carefully monitored following discontinuation of the SGLT-2 inhibitor and managed appropriately prior to surgery. The SGLT-2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved. Sick day rules apply Sick day rules PCDS |
|||
| Canagliflozin may increase the risk of lower-limb amputation (mainly toes) in patients with type 2 diabetes. Evidence does not show an increased risk for dapagliflozin and empagliflozin, but the risk may be a class effect. Preventive foot care is important for all patients with diabetes. See MHRA (March 2017) for SGLT2 inhibitors: updated advice on increased risk of lower-limb amputation (mainly toes). | |||
| If Fournier’s gangrene is suspected, stop the SGLT2 inhibitor and start treatment urgently (including antibiotics and surgical debridement). Fournier’s gangrene is a rare but potentially life-threatening infection that requires urgent medical attention. See MHRA (February 2019) for SGLT2 inhibitors: reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum). | |||
| The authorisation holder for dapagliflozin has withdrawn the indication for type 1 diabetes mellitus. The removal of the type 1 diabetes indication is not due to any new safety concerns and the other indications of dapagliflozin are unchanged. See MHRA (December 2021) for Dapagliflozin (Forxiga): no longer authorised for treatment of type 1 diabetes mellitus. | |||
| Dapagliflozin | 5mg tablet: £36.59 | For adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise - as monotherapy when metformin is considered inappropriate due to intolerance. - in addition to other medicinal products for the treatment of type 2 diabetes. For chronic heart failure with preserved or reduced ejection fraction. For the treatment of chronic kidney disease. The recommended dose is 10 mg dapagliflozin once daily. When dapagliflozin is used in combination with insulin or an insulin secretagogue, such as a sulphonylurea, a lower dose of insulin or insulin secretagogue may be considered to reduce the risk of hypoglycaemia. It is not recommended to initiate treatment with dapagliflozin in patients with an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73m2. In patients with severe hepatic impairment, a starting dose of 5 mg is recommended. If well tolerated, the dose may be increased to 10 mg. |
|
| 10mg tablet: £36.59 | |||
| Empagliflozin | 10mg tablet: £36.59 | For the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise - as monotherapy when metformin is considered inappropriate due to intolerance - in addition to other medicinal products for the treatment of diabetes. The recommended starting dose is 10 mg empagliflozin once daily for monotherapy and add-on combination therapy with other medicinal products for the treatment of diabetes. In patients tolerating empagliflozin 10 mg once daily who have an eGFR ≥60 ml/min/1.73 m2 and need tighter glycaemic control, the dose can be increased to 25 mg once daily. The maximum daily dose is 25 mg. For chronic heart failure with preserved or reduced ejection fraction and chronic kidney disease. The recommended dose is 10 mg empagliflozin once daily. When empagliflozin is used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia. If a dose is missed, it should be taken as soon as the patient remembers; however, a double dose should not be taken on the same day. It is not recommended to initiate treatment with empagliflozin in patients with an eGFR <20 ml/min/1.73 m2. It is not recommended in patients with severe hepatic impairment. |
|
| 25mg tablet: £36.59 | |||
| Canagliflozin | 100mg tablet: £39.20 (30) | For the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise: - as monotherapy when metformin is considered inappropriate due to intolerance or contraindications - in addition to other medicinal products for the treatment of diabetes. The recommended starting dose of canagliflozin is 100 mg once daily. In patients tolerating canagliflozin 100 mg once daily who have an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 or CrCl ≥ 60 mL/min and need tighter glycaemic control, the dose can be increased to 300 mg once daily. In patients with glomerular filtration rate (eGFR) 30 to <60 mL/min/1.73 m2, the recommended dose is 100mg daily. Should not be initiated <30 mL/min/1.73 m2, continue 100 mg for patients already taking. It is not recommended in patients with severe hepatic impairment. |
|
| 300mg tablet: £39.20 (30) | |||
| Ertugliflozin ▼ | 5mg tablet: £29.40 | For the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise: • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications. • in addition to other medicinal products for the treatment of diabetes. The recommended starting dose of ertugliflozin is 5 mg once daily. In patients tolerating ertugliflozin 5 mg once daily, the dose can be increased to 15 mg once daily if additional glycaemic control is needed. When ertugliflozin is used in combination with insulin or an insulin secretagogue, a lower dose of insulin or the insulin secretagogue may be required to reduce the risk of hypoglycaemia. In patients with volume depletion, correcting this condition prior to initiation of ertugliflozin is recommended. If a dose is missed, it should be taken as soon as the patient remembers. Patients should not take two doses on the same day. Initiation is not recommended in patients with an estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73 m2 or CrCl less than 45 mL/min. In patients with an eGFR ≥ 45 to < 60 mL/min/1.73 m2, should be initiated at 5 mg and up-titrated to 15 mg as needed for glycaemic control. Ertugliflozin should be discontinued when eGFR is persistently less than 30 mL/min/1.73 m2 or CrCl is persistently less than 30 mL/min. Ertugliflozin is not recommended with patients with severe hepatic impairment. |
|
| 15mg tablet: £29.40 | |||
| Sodium-glucose cotransporter-2 (SGLT-2) inhibitor / Biguanide combination products | The following cost effective SGLT-2 inhibitor/biguanide combination products are included in the formulary as an option where patients are already taking or will be switching to the individual agents. These also have the additional benefit of reducing carbon footprint and tablet burden. | ||
| Canagliflozin/ Metformin as Vokanamet | 50mg/850mg tablets: £39.20 (60) 50mg/1g tablets: £39.20 (60) | ||
| Dapagliflozin/ Metformin as Xigduo | 5mg/850mg tablets: £36.59 (56) 5mg/1g tablets: £36.59 (56) | ||
| Empagliflozin/ Metformin as Synjardy | 5mg/850mg tablets: 36.59 (56) 5mg/1g tablets: 36.59 (56) 12.5mg/850mg tablets: 36.59 (56) 12.5mg/1g tablets: 36.59 (56) | ||
| Biguanides/DPP-4 inhibitors (Gliptins) | Metformin/ Sitagliptin | 1g/50mg tablets: £13.33 (56) | For type 2 diabetes mellitus not controlled by metformin alone or by metformin in combination with either a sulfonylurea or pioglitazone or insulin. Adult: One twice daily. |
| Sulphonylureas | Gliclazide | 40mg tablets: £3.36 80mg tablets: £0.87 | NICE NG28 recommends prescribing a sulfonylurea with a low acquisition cost when a sulfonylurea is indicated. |
| Glitazones (thiazolidinediones) Prescribing of Pioglitazone should be in line with MHRA/EMEA advice ( Jan-11 & Aug-11) and NICE guidance NG28 Pioglitazone should not be started in people who: ○ are at higher risk of fracture ○ have evidence of heart failure The incidence of heart failure is increased when Pioglitazone is combined with insulin Inform patient of risk of oedema and what to do if this happens. Closely monitor patients during treatment with pioglitazone for signs and symptoms of fluid retention. Discontinue pioglitazone if heart failure develops. Following consideration at Somerset Prescribing Forum (Nov 2011) it was agreed that generic versions of pioglitazone may be used for all indications |
|||
| Thiazolidinediones | Pioglitazone | 15mg tablets: £8.23 30mg tablets: £10.89 45mg tablets: £11.71 | The PROACTIVE trial showed improvements in secondary outcomes. Pioglitazone is licensed for use with insulin Continue only if there is a reduction ≥ 0.5% points in HbA1c in 6 months Pioglitazone might be preferable to a DPP-4 inhibitor if there is marked insulin insensitivity, or if DPP-4 inhibitor is contraindicated or not tolerated. Secondary or tertiary centres may recommend the use of pioglitazone in patients with advanced liver fibrosis whether they have diabetes or not according to NICE NG 49 July 2016. This is an unlicensed use-please note. |
| DPP-4 inhibitors (Gliptins) | Somerset guidance Due to the established improved cardiovascular outcomes of SGLT-2s (gliflozins) we would recommend using the European (EASD) guidelines of using SGLT-2s in preference to gliptins whether or not a patient has a particular CVD risk. Generally only frail elderly patients do better on DPP-4s (gliptins) https://www.somersetccg.nhs.uk/wp-content/uploads/2021/08/The-use-of-Gliptins-in-the-management-of-type-2-diabetes-ASB-edit.pdf Related alert MHRA Dipeptidylpeptidase-4 inhibitors: risk of acute pancreatitis December 2014 Please note: NB Group 2 (LGV/PCV) drivers are required to notify DVLA if taking combination of gliptin with sulphonylurea See below for dose adjustment in renal impairment Please note that NICE does not support quadruple therapy, neither is this licensed by any of the oral medications |
||
| Sitagliptin | 25mg tablet: £4.83 | First line Sitagliptin is licensed for triple therapy with metformin & sulphonylurea and with insulin +/- metformin |
|
| 50mg tablet: £13.62 | |||
| 100mg tablet: £19.49 | |||
| Alogliptin | 6.25mg tablet: £26.60 | As a dual therapy add-on to other agents including insulin. Please note that the licence for alogliptin does not preclude any particular combination including triple therapy with metformin and a sulphonylurea (SU), and the use within this specific combination is not contraindicated. The safety and efficacy of alogliptin when used as triple therapy with metformin and a sulphonylurea have not been fully established. Monotherapy would be considered “off license” |
|
| 12.5mg tablet: £26.60 | |||
| 25mg tablet: £26.60 | |||
| Vildagliptin | 50mg tablet: £29.10 (56) | Twice daily dosing. In dual combination with a sulphonylurea, the recommended dose of vildagliptin is reduced to 50mg once daily administered in the morning. In this patient population, vildagliptin 100mg daily was no more effective than vildagliptin 50mg once daily |
|
| Saxagliptin | 2.5mg tablet: £31.60 | Saxagliptin is is licensed for triple therapy with metformin & sulphonylurea and with insulin +/- metformin | |
| 5mg tablet: £31.60 | |||
| Linagliptin | 5mg tablet: £33.26 | Monotherapy if metformin intolerant or contraindicated. Dual therapy with metformin. Triple therapy with SU and metformin. Can use with insulin with or without metformin. No dose adjustment needed for renal impairment |
|
| GLP-1 mimetic (Glucagon-like peptide-1 analogue) | Related references MHRA alert June 2019. GLP-1 receptor agonists: reports of diabetic ketoacidosis when concomitant insulin was rapidly reduced or discontinued Dietary advice should be given before starting therapy with these agents. NICE says :If triple therapy with metformin and 2 other oral drugs is not effective, not tolerated or contraindicated, consider combination therapy with metformin, a sulfonylurea and a glucagon-like peptide-1 (GLP-1) mimetic for adults with type 2 diabetes who: -have a BMI > 35 kg/m2 (adjust accordingly for people from black, Asian and other minority ethnic groups) and specific psychological or other medical problems associated with obesity or -have a BMI less than 35 kg/m2 for whom insulin therapy would have significant occupational implications or weight loss would benefit other significant obesity-related comorbidities. Only continue GLP-1 mimetic therapy if the person with type 2 diabetes has had a beneficial metabolic response (a reduction of at least 11 mmol/mol [1.0%] HbA1c and a weight loss of at least 3% of initial body weight in 6 months). NB Group 2 (LGV/PCV) drivers are required to notify DVLA if taking combination of exenatide with sulphonylurea. Somerset Prescribing Forum approved the use of exenatide as adjunctive therapy to basal insulin (with or without metformin and/or pioglitazone in adults with Type 2 diabetes) i.e. within the respective licensed indications. Patients are expected to show 0.5% (6mmol/mol) reduction in HbA1c after 6 months to justify continuation. Do not add GLP-1 treatment to basal insulin plus SU regimen as high risk of hypoglycaemia | ||
| Semaglutide as Ozempic® | 0.25mg/0.19ml solution for injection 1.5ml pre-filled disposable device: £73.25 (1) | NHS Somerset classify as green for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. Adult: initially 0.25 mg once weekly for 4 weeks, then increased to 0.5 mg once weekly for at least 4 weeks, then increased if necessary to 1 mg once weekly. Injections are once-weekly dosing. One pen lasts 4 weeks |
|
| 0.5mg/0.37ml solution for injection 1.5ml pre-filled disposable device: £73.25 (1) | |||
| 1mg/0.74ml solution for injection 3ml pre-filled disposable device: £73.25 (1) | |||
| NHS Somerset classify Semaglutide as Wegovy® as a Red drug (specialist prescribing only) for managing overweight and obesity and not recommended for managing overweight and obesity in young people aged 12 to 17 years or reduce the risk of major adverse cardiovascular events in adults with established cardiovascular disease and either obesity or overweight as per Traffic light guidance. | |||
| as Rybelsus® | 3mg tablet: £78.48 (30) | Oral semaglutide is very poorly absorbed and must be taken exactly as the manufacturers stipulate - must be swallowed whole on an empty stomach with a sip of water (up to half a glass of about 120mls) . No food or other medications should be taken for 30 minutes. Initially 3mg daily increasing after one month to 7mg once daily. After a further month increase to max 14mg once daily if required. Combination therapy, consider lower dose of sulfonylurea/insulin. |
|
| 7mg tablet: £78.48 (30) | |||
| 14mg tablet: £78.48 (30) | |||
| or | Dulaglutide Once-weekly dosing | 0.75mg, 1.5mg, 3mg and 4.5mg (0.5ml pre-filled pen): £73.25 (4 doses) | |
| or | Exenatide as Bydureon BCise® | 2mg/0.85ml prolonged-release suspension for injection pre-filled disposable device: £73.36 (4) | For subcutaneous administration once-weekly. |
| Second line | Liraglutide Once-daily dosing | 6mg/ml pre-filled pen: £78.48 (2 x 3ml), £117.72 (3 x 3ml) | |
| Glucose-dependent insulinotropic polypeptide receptor and Glucagon-like peptide-1 receptor agonist | Tirzepatide as Mounjaro Kwikpen® 1 Kwikpen LASTS 4 WEEKS | 2.5mg/0.6ml solution for injection 2.4ml pre-filled disposable device: £92.00 (1) | For treating type 2 diabetes alongside diet and exercise in adults when it is insufficiently controlled only if: • triple therapy with metformin and 2 other oral antidiabetic drugs is ineffective, not tolerated or contraindicated, and • they have a body mass index (BMI) of 35 kg/m2 or more, and specific psychological or other medical problems associated with obesity, or • they have a BMI of less than 35 kg/m2, and: - insulin therapy would have significant occupational implications, or - weight loss would benefit other significant obesity-related complications. Adult: Initially 2.5 mg once weekly for 4 weeks, then increased to 5 mg once weekly for at least 4 weeks, then increased if necessary up to 15 mg once weekly, dose to be increased in steps of 2.5 mg at intervals of at least 4 weeks. Tirzepatide (Mounjaro®) for weight loss will be made available by 23rd June 2025 to prioritised cohorts in primary care as part of an initial phased implementation period, which reflects the current capacity within primary care settings – Further information will be available in due course Obese or overweight female patients using oral contraceptives should consider also using a barrier method of contraception (e.g., a condom) or switching to a non-oral contraceptive method for 4 weeks after starting Mounjaro and for 4 weeks after each increase in dose as Mounjaro may affect how well the contraceptive pill works in these patients. |
| 5mg/0.6ml solution for injection 2.4ml pre-filled disposable device: £92.00 (1) | |||
| 7.5mg/0.6ml solution for injection 2.4ml pre-filled disposable device: £107.00 (1) | |||
| 10mg/0.6ml solution for injection 2.4ml pre-filled disposable device: £107.00 (1) | |||
Dose adjustments for empagliflozin in renal impairment
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Related guidance: NICE NG28 Type 2 diabetes in adults: management (2015 Updated December 2020) Type 1 diabetes in adults: diagnosis and management (July 2021 update) Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18) (2015 Updated December 2020) NICE type 2 adult pathway American Diabetes Association - Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes 2021 Management of Hyperglycemia in Type 2 Diabetes, 2019. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Please note that new NICE guidance for T1 and T2 diabetes is in development. |
|||
| The diabetes formulary for Somerset is a collaboration between CCG medicines management, secondary, community and primary care. The formulary is kept under review and reflects the latest guidelines. The goal is to support people with diabetes in maintaining quality of life and minimising the risk of complications. This requires an individualised approach centred around the person with diabetes. Principles - knowledge – understanding diabetes is key to making informed decisions about self-management - glycaemic control – appropriate glycaemic control reduces complications -cardiovascular risk factor management – aggressive but appropriate management reduces complications - regular follow-up – supports successful self-management Lifestyle management Lifestyle management is the cornerstone of diabetes care, including nutrition, physical activity, weight management, smoking cessation and psychological support. Before any pharmacological interventions are considered for people with type 2 diabetes, there should be a 3 month period of diet & lifestyle interventions. • Education - provide structured education to every patient and/or their carer at and around the time of diagnosis and review at least annually. • Diet - provide individualised and ongoing specialist nutritional advice. • Lifestyle - encourage weight loss, physical activity and smoking cessation Pharmacotherapy The expanding number of insulins and other glucose-lowering treatments and growing information about their benefits and risks provides more options for people with diabetes than ever, but can make decisions about the most appropriate option challenging. Support is available from the Somerset Diabetes Service, including - MyDiabetesMyWay (https://somerset.mydiabetes.com/ ) – an interactive website and smartphone app for people with diabetes and their family, friends and carers. It provides accurate information and resources, which are equally useful for healthcare professionals. Access to personal data, in addition to the information resources, empowers people with diabetes to self-manage. Advice, guidance and triage – a single point of access for non-urgent advice and all referrals into the diabetes service (except podiatry and dietetics). Queries will be answered within 3 working days. To make a referral open the EMIS document template, complete all the required fields and then email it to spn-tr.somersetdiabetesaandg@nhs.net DO NOT USE eReferrals. The response will appear in the documents inbox in EMIS via MESH. Virtual Clinics – non-patient facing case discussion meetings in primary care. The specialist team visits the practice to discuss a cohort of patients identified by the primary care team and to provide more general education as well as updates on the Somerset Diabetes Service. The clinics are co-ordinated by Leanne Dalley (Leanne.dalley@somersetFT.nhs.uk ) Before any pharmacological interventions are considered there should be a 3 month period of diet & lifestyle interventions. • Education provide structured education to every patient and/or their carer at and around the time of diagnosis and review annually. • Diet provide individualised and ongoing specialist nutritional advice. • Lifestyle encourage weight loss and exercise. Blood Pressure Control Evidence from UKPDS indicates that control of blood pressure in people with hypertension & Type 2 diabetes achieves a clinically important reduction in the risk of deaths related to diabetes, complications related to diabetes, progression of diabetic retinopathy, and deterioration in visual acuity. Blood glucose control The VADT, ACCORD and ADVANCE trials show that tight control of blood glucose in long standing Type 2 diabetics (reducing HbA1c to below 53mmol/mol or 7%) may be harmful. • Involve the person in decisions about their individual HbA1c target which may be above the general target of 48mmol/mol or 6.5% especially in long standing diabetes. • Offer lifestyle advice and medication to help achieve and maintain the HbA1c target. • Inform patients with a higher HbA1c that any reduction towards the agreed target is advantageous to their health. • Avoid pursuing highly intensive management to levels of <48mmol/mol or 6.5%. ♦ Self-monitoring of blood glucose should be offered to a patient newly diagnosed with T2DM only as an integral part of his/her self- management education. Its purpose should be discussed and there should be agreement how the results should be interpreted and acted upon. ♦ Eye and kidney damage should be screened annually. NB DH require that HbA1c should always be measured in millimoles per mol (mmol/mol) as well as by percentage. HbA1c of 6.5% is equivalent to 48mmol/mol. |
|||
| Self monitoring of blood glucose Do not routinely offer self-monitoring of blood glucose levels for adults with type 2 diabetes unless: the person is on insulin or there is evidence of hypoglycaemic episodes or the person is on oral medication that may increase their risk of hypoglycaemia while driving or operating machinery or the person is pregnant, or is planning to become pregnant. Which test strips should I prescribe? Somerset CCG recommends the use of devices which use strips not exceeding £9.25 per 50 strips. See section 6.1 Guidance on Blood Glucose Testing and Use of Test Strips for a full list of formulary approved strips. |
|||
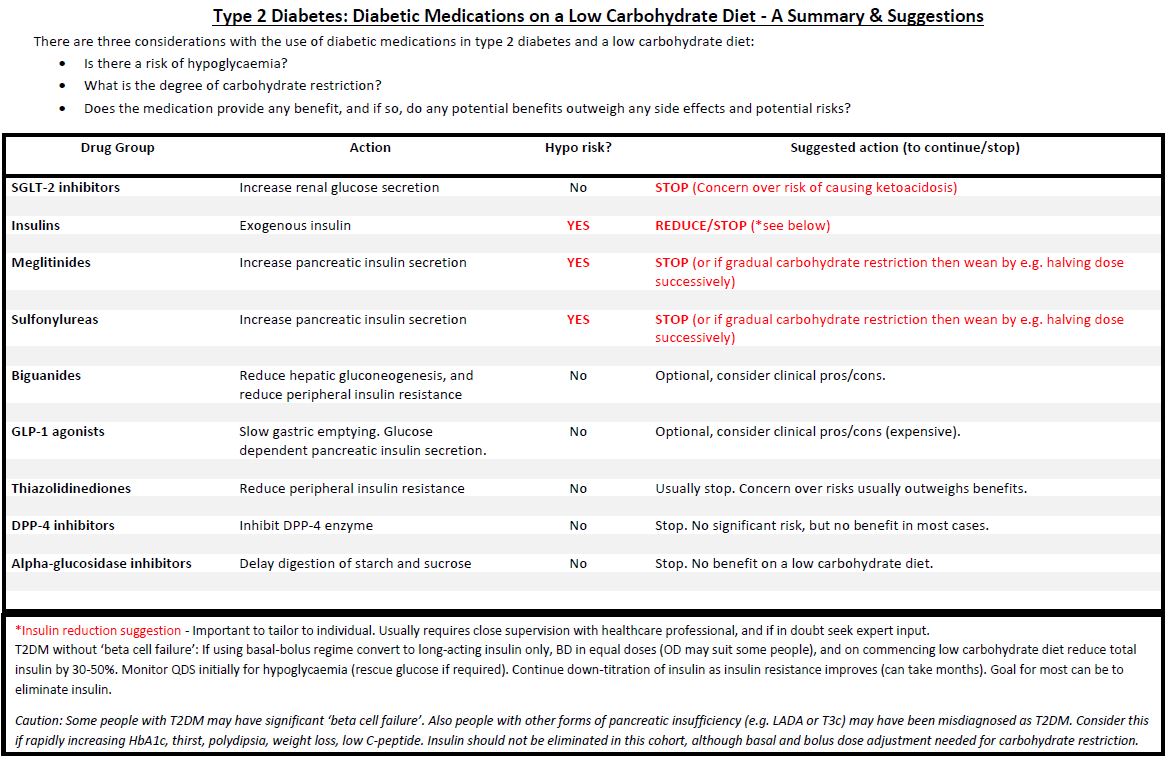
| Type 2 diabetes medications on a low carbohydrate diet. For patients who choose to follow a low carbohydrate diet to improve their blood glucose control, guidance has been drawn up by our diabetes principals on how medications could be optimised. Website link The Low Carb Program has now been accepted as an NHS approved app for Android and IOS available here |
|||