Related guidance:
Type 2 diabetes in adults: management NICE guideline (NG28 December 2015, updated June 2022)
Diabetes: treatment during pregnancy (SPS January 2022)
Diabetes mellitus: assessing fitness to drive (DVLA March 2016, updated June 2022)
Dr David Unwin’s sugar infographics (Public Health Collaboration)
- These sugar infographics help people understand the approximate effect various foods may have on their blood sugar in terms of a 4 gram teaspoon of sugar. For example, a bowl of 150g of boiled rice is roughly equivalent to ten teaspoons of table sugar.
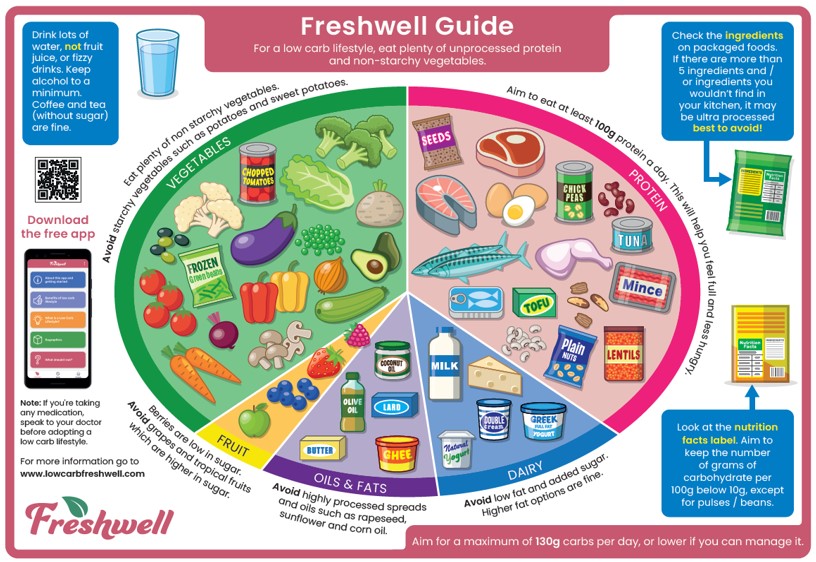
- The Freshwell Low Carb Project is an initiative set up by Dr David Oliver and Dr Kim Andrews at the Freshwell Health Centre, Essex.
- This project grew out of our concern about the rise in incidence of type 2 diabetes over the last few years, alongside the widely reported rapid increase in obesity in the UK population.
- Freshwell Low Carb Project -Meal Planners provides inspiration and recipes to make it a little easier to adopt a low carbohydrate lifestyle. All meal planners can be downloaded as a PDF for free and also available on the free Freshwell App.
| 6.4.1 - Blood glucose lowering drugs |
| 6.4.2 - Insulin, needles and lancets |
| 6.4.3 - Blood glucose testing and continuous glucose monitoring (CGM) |
See NHS Somerset Medicines Management Diabetes