Related guidance:
Chronic heart failure in adults: diagnosis and management NICE guideline (NG106 September 2018)
For Dapagliflozin see NHS Somerset Blood glucose lowering drugs
For Empaglifozin see NHS Somerset Blood glucose lowering drugs
Scenario: Confirmed heart failure with preserved ejection fraction (NICE CKS Heart failure – chronic: updated July 2023)Scenario: Confirmed heart failure with reduced ejection fraction (NICE CKS Heart failure – chronic: updated 2023)
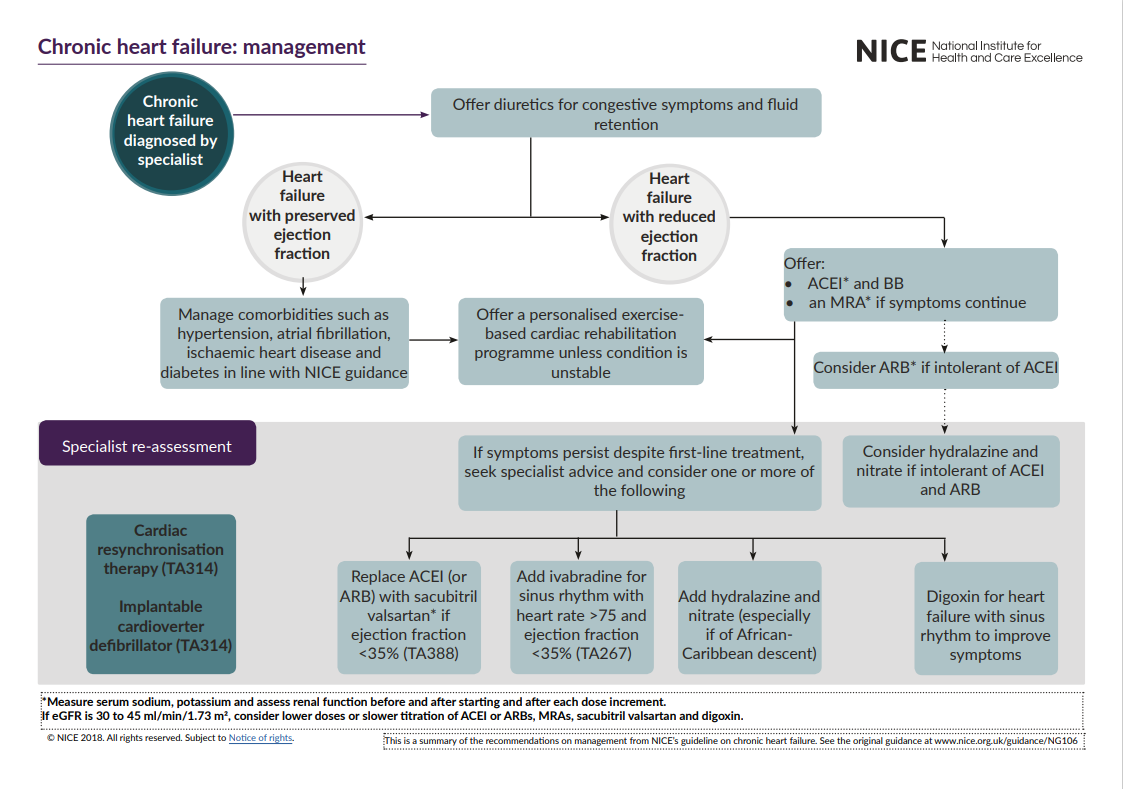
First-line treatment
Offer both angiotensin-converting enzyme (ACE) inhibitors and a beta-blocker (BB) licensed for heart failure to all patients with heart failure with reduced ejection fraction. Use clinical judgement when deciding which drug to start first.
ACE inhibitors
Do not offer ACE inhibitor therapy if there is a clinical suspicion of haemodynamically significant valve disease until the valve disease has been assessed by a specialist.
Start ACE inhibitor therapy at a low dose and titrate upwards at short intervals (for example, every 2 weeks) until the target or maximum tolerated dose is reached.
Measure serum sodium and potassium, and assess renal function, before and 1 to 2 weeks after starting an ACE inhibitor, and after each dose increment.
Measure blood pressure before and after each dose increment of an ACE inhibitor. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults.
Once the target or maximum tolerated dose of an ACE inhibitor is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell.
Formulary recommendation
- LISINOPRIL initially 2.5-5mg ONCE DAILY, titrated up to 30-35mg ONCE DAILY, or
- RAMIPRIL initially 2.5mg ONCE DAILY (1.25mg if already prescribed a diuretic), titrated up to 10mg ONCE DAILY
Monitoring: serum urea, creatinine, electrolytes and eGFR at initiation and after each dose increment
ACE inhibitor prescribing supporting information
Alternative treatments if ACE inhibitors are not tolerated
Consider an ARB (sartan) licensed for heart failure as an alternative to an ACE inhibitor for people who have heart failure with reduced ejection fraction and intolerable side effects with ACE inhibitors.
Measure serum sodium and potassium, and assess renal function, before and after starting an ARB and after each dose increment.
Measure blood pressure after each dose increment of an ARB. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults.
Once the target or maximum tolerated dose of an ARB is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell.
If neither ACE inhibitors nor ARBs are tolerated, seek specialist advice and consider hydralazine in combination with nitrate for people who have heart failure with reduced ejection fraction.
Formulary recommended ARB;
- LOSARTAN initially 12.5mg ONCE DAILY, increased at 1 – 2 weekly intervals, to 50mg ONCE DAILY
- CANDESARTAN initially 4mg ONCE DAILY, doubling the dose at intervals of no less than 2 weeks, to 32mg ONCE DAILY
Monitoring: serum potassium, creatinine, & eGFR required at baseline, week 2, week 4, and monthly thereafter
ARB (A2RA, sartans) prescribing support information
Beta-blockers
Do not withhold treatment with a beta-blocker solely because of age or the presence of peripheral vascular disease, erectile dysfunction, diabetes, interstitial pulmonary disease or chronic obstructive pulmonary disease.
Introduce beta-blockers in a ‘start low, go slow’ manner. Assess heart rate and clinical status after each titration. Measure blood pressure before and after each dose increment of a beta‑blocker. .
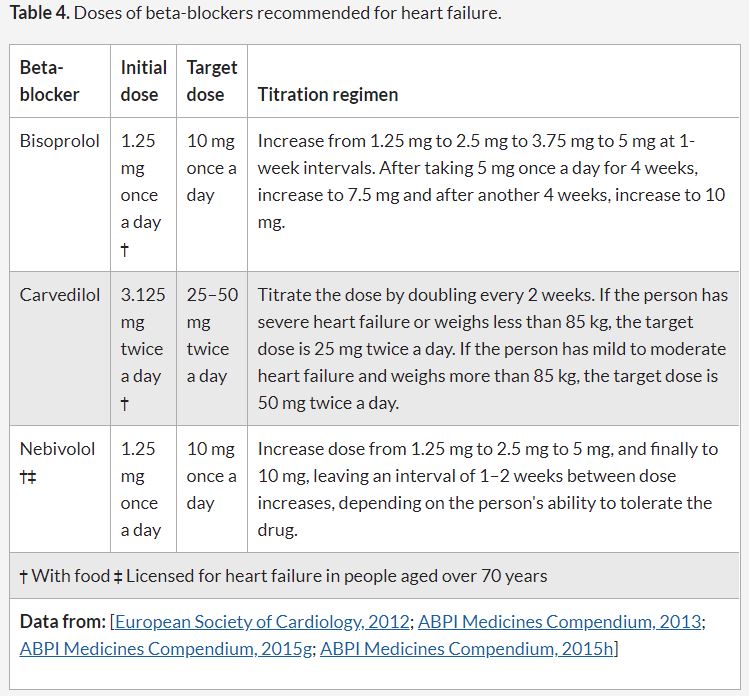
The beta-blockers licensed in the UK for the treatment of heart failure are bisoprolol, carvedilol, and nebivolol.
- Nebivolol is licensed for stable mild to moderate heart failure in people aged 70 years or older.
- People with stable symptoms who are receiving treatment with a beta-blocker for a concomitant condition (for example angina or hypertension) who develop heart failure with reduced ejection fraction (HF-REF) should switch their current beta-blocker to a beta-blocker licensed for treating heart failure.
Recommended formulary choice
BISOPROLOL initially 1.25mg ONCE DAILY, titrated according to response and tolerability to 10mg ONCE DAILY as below
Beta blocker prescribing support information
Mineralocorticoid receptor antagonists
Offer an MRA, (eplerenone,spironolactone) in addition to an ACE inhibitor (or ARB) and beta-blocker, to people who have heart failure with reduced ejection fraction if they continue to have symptoms of heart failure.
Measure serum sodium and potassium, and assess renal function, before and after starting an MRA and after each dose increment.
Measure blood pressure before and after after each dose increment of an MRA. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults.
Once the target, or maximum tolerated, dose of an MRA is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell.
For further specialist treatments see here
Dapagliflozin
A positive NICE appraisal of dapagliflozin in December 2020 https://www.nice.org.uk/guidance/ta679/documents/final-appraisal-determination-document
- Dapagliflozin is recommended as an option for treating symptomatic chronic heart failure with reduced ejection fraction in adults, only if it is used as an add-on to optimised standard care.
- Start treatment of symptomatic heart failure with reduced ejection fraction with dapagliflozin on the advice of a heart failure specialist. Monitoring should be done by the most appropriate healthcare professional.
Empagliflozin
Empagliflozin is recommended as an option for treating symptomatic chronic heart failure with reduced ejection fraction in adults, only if it is used as an add-on to optimised standard care with:
- an angiotensin-converting enzyme (ACE) inhibitor or angiotensin 2 receptor blocker (ARB), with a beta blocker and, if tolerated, a mineralocorticoid receptor antagonist (MRA), or
- sacubitril valsartan with a beta blocker and, if tolerated, an MRA.
Start empagliflozin for treating symptomatic heart failure with reduced ejection fraction on the advice of a heart failure specialist. Monitoring should be done by the most appropriate healthcare professional.