| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| The following advice on respiratory prescribing is based on SIGN/BTS gudaince or NICE guidance. All inhalers except very high potency steroids are included for prescribing in primary care setting, and where possible we highlight the best value inhalers in each category. We support the preferential use of Dry Powder Inhalers (DPIs) over pressurised metered dose inhalers (pMDIs) whwever possible based on the patients ability to use | |||
| Written personalised action plans as part of self-management education have been shown to improve health outcomes for people with asthma and should be offered to all patients. Asthma Self Care Plan on NHS Somerset website | |||
| Note on inhaler devices • Patients ability to use different devices varies; assessment of response to a prescribed treatment should include evaluation of inhaler technique as demonstrated by the patient • A spacer device should be used with all doses of 1000mcg or higher BDP equivalent • Aerochamber Plus® spacer (medium-volume) has a flexible gasket & should fit all MDIs however SPC should be checked to ensure device is compatible Correct inhaler technique is vital to ensure maximum benefit is obtained from inhaled therapies. Because the force of inhalation is different for MDI and dry powder inhalers, where possible patients should have just one type of device i.e. all MDI or all DPI (see Types of Inhaler patient information leaflet) |
|||
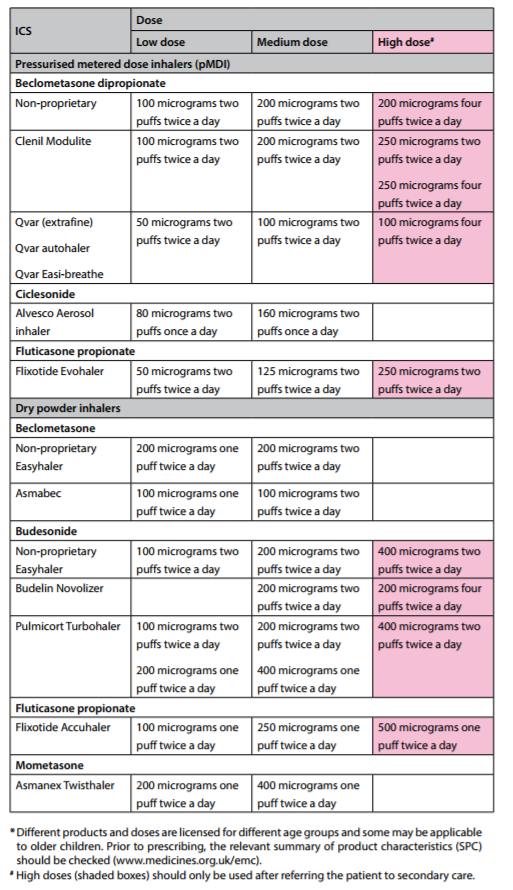
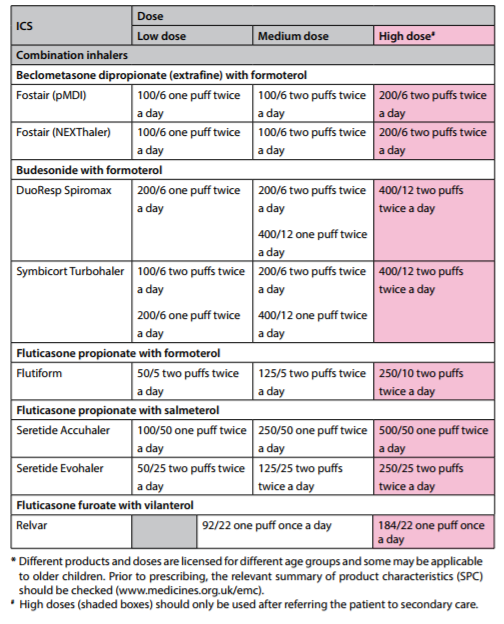
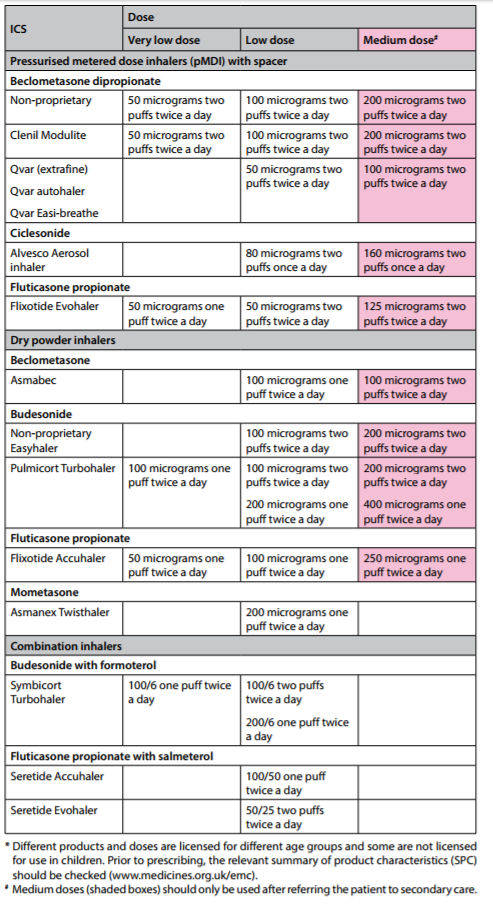
| Safer use of inhaled corticosteroids (ICS) • ALL patients on high dose ICS (ie >= 1000mcg beclometasone or equivalent daily) should be issued with a Steroid Warning Card, and also use a spacer device if using a pMDI inhaler. • ICS can have serious side effects: one study has shown an increased risk of diabetes onset and progression. The risk increased with higher doses – patients on ICS doses of 1000mcg fluticasone/day or more (2000mcg beclometasone) had a 64% increased risk of developing diabetes compared to no ICS use. • Stepping-down asthma therapy helps reduce the ICS dose and can be considered in patients with complete asthma control (for at least 12 weeks). For patients on combination therapy the preferred approach is to reduce the ICS by approximately 50% while continuing LABA at the same dose initially. See the asthma step-down guide for combination ICS/LABA inhalers p.71/55 • Different ICS have different potencies and the equivalent dose can also vary between devices. Standard practice was to express the dose equivalent to beclometasone dipropionate as shown below. Weight for weight, the inhaled steroid in Qvar®, Fostair® and all fluticasone inhalers deliver a much higher steroid dose. REDUCE DOSE ACCORDINGLY IN THESE PREPARATIONS. 400mcg Clenil Modulite® or Soprobec® = 400mcg BDP 200mcg Qvar® or Kelhale® = 400-500mcg BDP 200mcg Fostair® = 500mcg BDP 400mcg budesonide (Pulmicort®/Symbicort®/Easyhaler®) = 400mcg BDP 200mcg fluticasone (Flixotide®/Seretide®/Flutiform®) = 400mcg BDP |
|||
| Latest BTS asthma guidance moves away from measuring corticosteroid potency in beclometasone (BDP) equivalent and suggests using bands of potency. Essentially, as a conversion: Very low dose (children): 200mcg BDP/day Low dose: 400mcg Medium dose: 800mcg High dose: 1000mcg and above. Refer for specialist care before prescribing 1000mcg |
|||
Adult inhalers