13.3.1 Eczema and psoriasis
Related guidance:
British Association of Dermatologists
For Steroid Emergency Card see NHS Somerset Formulary Corticosteriod responsive conditions.
For emollients see NHS Somerset Formulary Dry and scaling skin disorders
For antihistamines see NHS Somerset Formulary Allergic conditions
Items which should not be routinely prescribed in primary care
Silk garments
- Silk garments are typically prescribed for eczema or dermatitis.
- These products are knitted, medical grade silk clothing that can be used as an adjunct to normal treatment for severe eczema and allergic skin conditions.
- Four brands of knitted silk garments are currently listed as an appliance in part IX A in the NHS Drug Tariff and are relatively expensive.
- The PrescQIPP document on silk garments states that the evidence relating to their use is weak and is of low quality.
- In addition, due to limited evidence supporting the efficacy of silk clothing for the relief of eczema, the NIHR HTA programme commissioned the CLOTHES trial, to examine whether adding silk garments to standard eczema care reduced eczema severity in children with moderate to severe eczema, compared to use of standard eczema treatment alone. The trial concluded that using silk garments for the management of eczema is unlikely to be cost-effective for the NHS.
- NHS England recommend that GPs:
• Do not initiate.
• Deprescribe in patients currently prescribed this medicine.
Psoriasis: assessment and management Clinical guideline (CG153 October 2012, updated September 2017)
Eczema in under 12s
Identification and management of trigger factors
- When clinically assessing children with atopic eczema, healthcare professionals should seek to identify potential trigger factors including:
- irritants, for example soaps and detergents (shampoos, bubble baths, shower gels and washing-up liquids)
- skin infections
- contact allergens
- food allergens
- inhalant allergens. - Should consider a diagnosis of food allergy in children with atopic eczema who have reacted previously to a food with immediate symptoms, or in infants and young children with moderate or severe atopic eczema that has not been controlled by optimum management, particularly if associated with gut dysmotility (colic, vomiting, altered bowel habit) or failure to thrive.
- Should provide assurance that most children with mild atopic eczema do not need to have tests for allergies and advise against high street or internet allergy tests because there is no evidence of their value in the management of atopic eczema.
- Should offer a 6 to 8 week trial of an extensively hydrolysed protein formula or amino acid formula in place of cow’s milk formula for bottle-fed infants aged under 6 months with moderate or severe atopic eczema that has not been controlled by optimal treatment with emollients and mild topical corticosteroids and should refer children who follow a cow’s milk-free diet for longer than 8 weeks for specialist dietary advice. Diets based on unmodified proteins of other species’ milk (for example goat’s milk, sheep’s milk) or partially hydrolysed formulas should not be used in children with atopic eczema for the management of suspected cow’s milk allergy. Diets including soya protein can be offered to children aged 6 months or over with specialist dietary advice. Inform women who are breastfeeding children with atopic eczema that it is not known whether altering the mother’s diet is effective in reducing the severity of the condition. A trial of an allergen-specific exclusion diet should be considered under dietary supervision if food allergy is strongly suspected.
- It is unclear what role factors such as stress, humidity or extremes of temperature have in causing flares of atopic eczema. These factors should be avoided where possible.
- Healthcare professionals should be aware that all categories of severity of atopic eczema, even mild, can have a negative impact on psychological and psychosocial wellbeing and quality of life. This should be taken into account when deciding on treatment strategies and should consider using the following additional tools to provide objective measures of the severity of atopic eczema, quality of life and response to treatment: visual analogue scales (0 to 10) capturing the child’s and/or parents’ or carers’ assessment of severity, itch and sleep loss over the previous 3 days and nights, validated tools:
Patient-Oriented Eczema Measure (POEM) for severity See University of Nottingham website
Children’s Dermatology Life Quality Index (CDLQI) See Cardiff University website
Infants’ Dermatitis Quality of Life Index (IDQoL) See Cardiff University website
Dermatitis Family Impact (DFI) questionnaire for quality of life See Cardiff University website
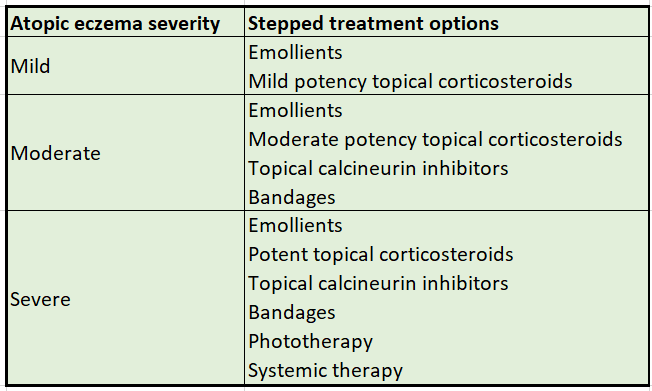
Treatment
- Emollients should form the basis of atopic eczema management and should always be used, even when the atopic eczema is clear. Management can then be stepped up or down, according to the severity of symptoms.Treatment for flares should be started as soon as signs and symptoms appear and continued for approximately 48 hours after symptoms subside. See Dry and scaling skin disorders in NHS Somerset formulary for emollients.
- Emollient creams are vital in helping to manage dry skin conditions but there are warnings about the risk of severe and fatal burns being extended to all paraffin-based emollients regardless of paraffin concentration. Data suggest there is also a risk for paraffin-free emollients. Advise patients who use these products not to smoke or go near naked flames, and warn about the easy ignition of clothing, bedding, dressings, and other fabric that have dried residue of an emollient product on them. See Emollients: new information about risk of severe and fatal burns with paraffin-containing and paraffin-free emollients (MHRA December 2018)
- Healthcare professionals should offer children with atopic eczema and their parents or carers information on how to recognise flares of atopic eczema (increased dryness, itching, redness, swelling and general irritability) and given clear instructions on how to manage flares according to the stepped-care plan.
- Should offer a choice of unperfumed emollients to use every day for moisturising, washing and bathing. This should be suited to the child’s needs and preferences, and may include a combination of products or one product for all purposes. Leave-on emollients should be prescribed in large quantities (250g to 500g weekly) and easily available to use at nursery, pre-school or school.
- Emollients should be smoothed onto the skin rather than rubbing them in.
- Offer an alternative emollient if a particular emollient causes irritation or is not acceptable to a child with atopic eczema.
- Review repeat prescriptions of individual products and combinations of products at least once a year to ensure that therapy remains optimal.
- Where emollients (excluding bath emollients) and other topical products are used at the same time of day, the different products should ideally be applied one at a time with several minutes between applications where practical.
- Oral antihistamines should not be used routinely in the management of atopic eczema in children.
- Should offer a 1 month trial of a non-sedating antihistamine to children with severe atopic eczema or children with mild or moderate atopic eczema where there is severe itching or urticaria. Treatment can be continued, if successful, while symptoms persist, and should be reviewed every 3 months. Should offer a 7 to 14 day trial of an age appropriate sedating antihistamine to children aged 6 months or over during an acute flare of atopic eczema if sleep disturbance has a significant impact on the child or parents or carers. This treatment can be repeated during subsequent flares if successful. See Allergic conditions in NHS Somerset formulary for antihistmines.
Topical corticosteriods
Several potent topical corticosteroid preparations are available in the UK, and the age from which they are licensed for use in children varies.
- Should discuss the benefits and harms of treatment with topical corticosteroids emphasising that the benefits outweigh possible harms when they are applied correctly.
Topical corticosteroids for atopic eczema should be prescribed for application only once or twice daily.
- The potency of topical corticosteroids should be tailored to the severity of the child’s atopic eczema, which may vary according to body site. Use
- mild potency for mild atopic eczema
- moderate potency for moderate atopic eczema
- potent for severe atopic eczema
- mild potency for the face and neck, except for short-term (3 to 5 days) use of moderate potency for severe flares
- moderate or potent for short periods only (7 to 14 days) for flares in vulnerable sites such as axillae and groin
Do not use very potent preparations in children aged under 12 months without specialist dermatological advice.
- Where more than 1 alternative topical corticosteroid is considered clinically appropriate within a potency class, the drug with the lowest acquisition cost should be prescribed, taking into account pack size and frequency of application.
- Only apply topical corticosteroids to areas of active atopic eczema (or eczema that has been active within the past 48 hours which may include areas of broken skin.
- Exclude secondary bacterial or viral infection if a mild or moderately potent topical corticosteroid has not controlled the atopic eczema within 7 to 14 days. If this treatment does not control the atopic eczema, the diagnosis should be reviewed and the child referred for specialist dermatological advice.
- Healthcare professionals who dispense topical corticosteroids should apply labels stating the potency class of the preparations to the container (for example, the tube), not the outer packaging.
- Should consider treating problem areas of atopic eczema with topical corticosteroids for 2 consecutive days per week to prevent flares, instead of treating flares as they arise, in children with frequent flares (2 or 3 per month), once the eczema has been controlled. This strategy should be reviewed within 3 months to 6 months to assess effectiveness.
- A different topical corticosteroid of the same potency should be considered as an alternative to stepping up treatment if tachyphylaxis to a topical corticosteroid is suspected.
How should topical corticosteroids be applied?
- A thin layer of topical corticosteroid should be applied once or twice daily, adjusting the potency to control symptoms. For many conditions, once daily application is usually sufficient. Increase to twice daily application only if the condition does not respond adequately.
- Avoid the use of the phrase ‘use sparingly’ to avoid undertreatment.
- Topical corticosteroids should typically be used in bursts of 3–7 days in order to achieve control. Once a clinical response is seen, withdraw the corticosteroid gradually.
- If an emollient is also being used, advise the person to wait 20–30 minutes between applications.
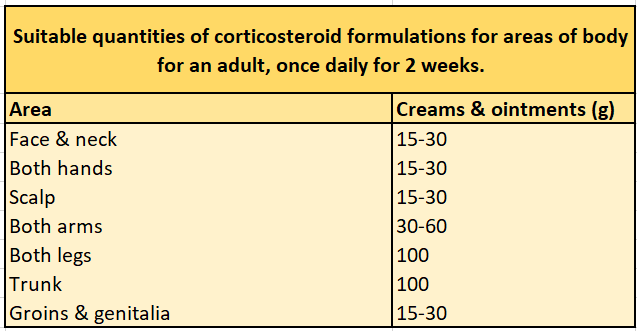
- Most products are supplied with an information leaflet specifying the number of fingertip units (FTUs) needed to treat specific body areas.
- One FTU is the length of cream or ointment expelled from a tube with a standard 5 mm diameter nozzle equivalent to the distance from the tip of the adult index finger to the first crease. One FTU is about 500 mg and is sufficient to treat a skin area about twice that of the flat of the hand with the fingers together.
- See the sections on Fingertip units (FTUs) for adults and Fingertip units (FTUs) for children for the approximate amount of topical corticosteroid that should be applied to different areas of the body.
Topical calcineurin inhibitors
- Topical tacrolimus and pimecrolimus are not recommended for the treatment of mild atopic eczema or as first-line treatments for atopic eczema of any severity.
NHS Somerset classify as black (not recommended) for mild or first line for any severity as per Traffic light guidance.
- Topical tacrolimus is recommended as an option for the second-line treatment of moderate to severe atopic eczema (adults and children aged 2 years and older) and topical pimecrolimus is recommended as an option for the second-line treatment of moderate atopic eczema (face and neck in children aged 2 years to 16 years) that has not been controlled by topical corticosteroids, where there is a serious risk of important adverse effects from further topical corticosteroid use, particularly irreversible skin atrophy.
NHS Somerset classify as amber for second line option as per Traffic light guidance.
- Topical calcineurin inhibitors should only be applied to areas of active atopic eczema, which may include areas of broken skin and should not be used under occlusion (bandages and dressings) without specialist dermatological advice.
- For facial atopic eczema in children that requires long-term or frequent use of mild topical corticosteroids, consider stepping up treatment to topical calcineurin inhibitors.
Dry bandages and medicated dressings including wet wrap therapy
- Occlusive medicated dressings and dry bandages should not be used to treat infected atopic eczema in children.
- Localised medicated dressings or dry bandages can be used with emollients as a treatment for areas of chronic lichenified (localised skin thickening) atopic eczema in children.
- Localised medicated dressings or dry bandages with emollients and topical corticosteroids can be used for short-term treatment of flares (7 to 14 days) or areas of chronic lichenified atopic eczema in children.
- Whole-body (limbs and trunk) occlusive dressings (including wet wrap therapy) and whole-body dry bandages (including tubular bandages and garments) should not be used as first-line treatment for atopic eczema in children and should only be initiated by a healthcare professional trained in their use.
- Whole-body (limbs and trunk) occlusive dressings (including wet wrap therapy) with topical corticosteroids should only be used to treat atopic eczema in children for 7 to 14 days (or for longer with specialist dermatological advice), but can be continued with emollients alone until the atopic eczema is controlled.
Managing infections
- Should be offered information on how to recognise the symptoms and signs of bacterial infection and how to access appropriate treatment when a child’s atopic eczema becomes infected.
- Should obtain new supplies of topical atopic eczema medications after treatment for infected atopic eczema because products in open containers can become contaminated with microorganisms and act as a source of infection.
- Should consider infection with herpes simplex (cold sore) virus if a child’s infected atopic eczema fails to respond to treatment with antibiotics and an appropriate topical corticosteroid. If suspected to be herpes simplex virus, treatment with oral aciclovir should be started even if the infection is localised.
- If eczema herpeticum (widespread herpes simplex virus) is suspected in a child with atopic eczema, treatment with systemic aciclovir should be started immediately and the child should be referred for same-day specialist dermatological advice.
- If secondary bacterial infection is also suspected, treatment with appropriate systemic antibiotics should also be started.
- If eczema herpeticum involves the skin around the eyes, the child should be treated with systemic aciclovir and should be referred for same-day ophthalmological and dermatological advice.
See patient information leaflet on Atopic eczema (British Association of Dermatologists May 2022)
Phototherapy and systemic treatments
- Healthcare professionals should consider phototherapy or systemic treatments for the treatment of severe atopic eczema in children when other management options have failed or are inappropriate and where there is a significant negative impact on quality of life. Treatment should be undertaken only under specialist dermatological supervision by staff who are experienced in dealing with children and should only be initiated in children with atopic eczema after assessment and documentation of severity of atopic eczema and quality of life.
Complementary therapies
- Children with atopic eczema and their parents or carers should be informed that the effectiveness and safety of complementary therapies such as homeopathy, herbal medicine, massage and food supplements for the management of atopic eczema have not yet been adequately assessed in clinical studies. Should be cautious with the use of herbal medicines in children and be wary of any herbal product that is not labelled in English or does not come with information about safe usage. See Using herbal medicines: advice to consumers (MHRA February 2008). Topical corticosteroids are deliberately added to some herbal products intended for use in children with atopic eczema and liver toxicity has been associated with the use of some Chinese herbal medicines intended to treat atopic eczema. Children with atopic eczema and their parents or carers should be asked if they are using or intend to use complementary therapies and informed that if they plan to use complementary therapies, they should keep using emollients as well and that regular massage with emollients may improve the atopic eczema.
Psoriasis
- Offer people with psoriasis topical therapy as first-line treatment.
- Offer second- or third-line treatment options (phototherapy or systemic therapy) at the same time when topical therapy alone is unlikely to adequately control psoriasis, such as:
• extensive disease (for example, more than 10% of body surface area affected) or
• at least ‘moderate’ on the static Physician’s Global Assessment or
• where topical therapy is ineffective, such as nail disease. - Offer practical support and advice about the use and application of topical treatments. Advice should be provided by healthcare professionals who are trained and competent in the use of topical therapies.
- When offering topical agents:
• take into account patient preference, cosmetic acceptability, practical aspects of application and the site(s) and extent of psoriasis to be treated.
• discuss the variety of formulations available and, depending on the person’s preference, use:
- cream, lotion or gel for widespread psoriasis
- lotion, solution or gel for the scalp or hair-bearing areas
- ointment to treat areas with thick adherent scale
• be aware that topical treatment alone may not provide satisfactory disease control, especially in people with psoriasis that is extensive (for example, more than 10% of body surface area affected) or at least ‘moderate’ on the static Physician’s Global Assessment. - If a person of any age with psoriasis requiring topical therapy has a physical disability, or cognitive or visual impairment offer advice and practical support that take into account the person’s individual needs.
- Arrange a review appointment 4 weeks after starting a new topical treatment in adults, and 2 weeks after starting a new topical treatment in children, to:
• evaluate tolerability, toxicity, and initial response to treatment
• reinforce the importance of adherence when appropriate
• reinforce the importance of a 4 week break between courses of potent/very potent corticosteroids
If there is little or no improvement at this review, discuss the next treatment option with the person. - Discuss with people whose psoriasis is responding to topical treatment (and their families or carers where appropriate):
• the importance of continuing treatment until a satisfactory outcome is achieved (for example, clear or nearly clear) or up to the recommended maximum treatment period for corticosteroids
• that relapse occurs in most people after treatment is stopped
• that after the initial treatment period topical treatments can be used when needed to maintain satisfactory disease control. - Offer people with psoriasis a supply of their topical treatment to keep athome for the self-management of their condition.
- In people whose psoriasis has not responded satisfactorily to a topical treatment strategy, before changing to an alternative treatment:
• discuss with the person whether they have any difficulties with application, cosmetic acceptability or tolerability and where relevant offer an alternative formulation
• consider other possible reasons for non-adherence
How to use corticosteroids safely
Several potent topical corticosteroid preparations are available in the UK, and the age from which they are licensed for use in children varies.
- Be aware that continuous use of potent or very potent corticosteroids may cause:
• irreversible skin atrophy and striae
• psoriasis to become unstable
• systemic side effects when applied continuously to extensive psoriasis (for example, more than 10% of body surface area affected).
Explain the risks of these side effects to people undergoing treatment (and their families or carers where appropriate) and discuss how to avoid them. - Aim for a break of 4 weeks between courses of treatment with potent or very potent corticosteroids. Consider topical treatments that are not steroid based (such as vitamin D or vitamin D analogues or coal tar) as needed to maintain psoriasis disease control during this period.
- When offering a corticosteroid for topical treatment select the potency and formulation based on the person’s need.
- Do not use very potent corticosteroids continuously at any site for longer than 4 weeks.
- Do not use potent corticosteroids continuously at any site for longer than 8 weeks.
- Do not use very potent corticosteroids in children and young people.
- Offer a review at least annually to adults with psoriasis who are using intermittent or short-term courses of a potent or very potent corticosteroid (either as monotherapy or in combined preparations) to assess for the presence of steroid atrophy and other adverse effects.
- Offer a review at least annually to children and young people with psoriasis who are using corticosteroids of any potency (either as monotherapy or in combined preparations) to assess for the presence of steroid atrophy and other adverse effects.
Calcitriol and tacalcitol preparations available in the UK are not licensed for use in children.
Different topical calcipotriol preparations are available in the UK, which vary in their licensing status for use in children and young people under 18.
Topical treatment of psoriasis affecting the trunk and limbs
- Offer a potent corticosteroid applied once daily plus vitamin D or a vitamin D analogue applied once daily (applied separately, 1 in the morning and the other in the evening) for up to 4 weeks as initial treatment for adults with trunk or limb psoriasis.
- If once-daily application of a potent corticosteroid plus once-daily application of vitamin D or a vitamin D analogue does not result in clearance, near clearance or satisfactory control of trunk or limb psoriasis in adults after a maximum of 8 weeks, offer vitamin D or a vitamin D analogue alone applied twice daily.
- If twice-daily application of vitamin D or a vitamin D analogue does not result in clearance, near clearance or satisfactory control of trunk or limb psoriasis in adults after 8 to 12 weeks, offer either:
• a potent corticosteroid applied twice daily for up to 4 weeks or
• a coal tar preparation applied once or twice daily. - If a twice-daily potent corticosteroid or coal tar preparation cannot be used or a once-daily preparation would improve adherence in adults offer a combined product containing calcipotriol monohydrate and betamethasone dipropionate applied once daily for up to 4 weeks.
- Offer treatment with very potent corticosteroids in adults with trunk or limb psoriasis only:
• in specialist settings under careful supervision
• when other topical treatment strategies have failed
• for a maximum period of 4 weeks. - Consider short-contact dithranol for treatment-resistant psoriasis of the trunk or limbs and either:
• give educational support for self-use or
• ensure treatment is given in a specialist setting.
For children and young people with trunk or limb psoriasis consider either:
- calcipotriol applied once daily (only for those over 6 years of age) or
- a potent corticosteroid applied once daily (only for those over 1 year of age).
Topical treatment of psoriasis affecting the scalp
- Offer a potent corticosteroid applied once daily for up to 4 weeks as initial treatment for people with scalp psoriasis.
- Show people with scalp psoriasis (and their families or carers where appropriate) how to safely apply corticosteroid topical treatment.
- If treatment with a potent corticosteroid does not result in clearance, near clearance or satisfactory control of scalp psoriasis after 4 weeks consider:
• a different formulation of the potent corticosteroid (for example, a shampoo or mousse) and/or
• topical agents to remove adherent scale (for example, agents containing salicylic acid, emollients and oils) before application of the potent corticosteroid. - If the response to treatment with a potent corticosteroid for scalp psoriasis remains unsatisfactory after a further 4 weeks of treatment offer:
• a combined product containing calcipotriol monohydrate and betamethasone dipropionate applied once daily for up to 4 weeks or
• vitamin D or a vitamin D analogue applied once daily (only in those who cannot use steroids and with mild to moderate scalp psoriasis). - If continuous treatment with either a combined product containing calcipotriol monohydrate and betamethasone dipropionate applied once daily or vitamin D or a vitamin D analogue applied once daily for up to 8 weeks does not result in clearance, near clearance or satisfactory control of scalp psoriasis offer:
• a very potent corticosteroid applied up to twice daily for 2 weeks for adults only or
• coal tar applied once or twice daily or
• referral to a specialist for additional support with topical applications and/or advice on other treatment options. - Consider topical vitamin D or a vitamin D analogue alone for the treatment of scalp psoriasis only in people who:
• are intolerant of or cannot use topical corticosteroids at this site or
• have mild to moderate scalp psoriasis. - Do not offer coal tar-based shampoos alone for the treatment of severe scalp psoriasis.
Topical treatment of psoriasis affecting the face, flexures and genitals
- Offer a short-term mild or moderate potency corticosteroid applied once or twice daily (for a maximum of 2 weeks) to people with psoriasis of the face, flexures or genitals.
- Be aware that the face, flexures and genitals are particularly vulnerable to steroid atrophy and that corticosteroids should only be used for short term treatment of psoriasis (1 to 2 weeks per month). Explain the risks to people undergoing this treatment (and their families or carers where appropriate) and how to minimise them.
- For adults with psoriasis of the face, flexures or genitals if the response to short-term moderate potency corticosteroids is unsatisfactory, or they require continuous treatment to maintain control and there is serious risk of local corticosteroid-induced side effects, offer a calcineurin inhibitor applied twice daily for up to 4 weeks. Calcineurin inhibitors should be initiated by healthcare professionals with expertise in treating psoriasis.
- Do not use potent or very potent corticosteroids on the face, flexures or genitals.
- When prescribing topical agents at facial, flexural and genital sites take into account that they may cause irritation and inform people undergoing treatment (and their families and carers where appropriate) of these risks and how to minimise them.
Systemic therapy
- Responsibility for use of systemic therapy should be in specialist settings only. Certain aspects of supervision and monitoring may be delegated to other healthcare professionals and completed in non-specialist settings,in which case, such arrangements should be formalised. When offering systemic therapy, tailor the choice of agent and dosing schedule to the needs of the individual and include consideration of:
• the person’s age
• disease phenotype, pattern of activity and previous treatment history
• disease severity and impact
• the presence of psoriatic arthritis (in consultation with a rheumatologist)
• conception plans
• comorbidities
• the person’s views. - Be aware of the benefits of, contraindications to and adverse effects associated with systemic treatments. Explain the risks and benefits to people undergoing this treatment (and their families or carers where appropriate), using absolute risks and natural frequencies when possible. Support and advice should be provided by healthcare professionals who are trained and competent in the use of systemic therapies.
- When reviewing response to systemic therapy, take into account:
• disease severity compared with baseline (for example, PASI baseline to endpoint score)
• control of psoriatic arthritis disease activity (in consultation with a rheumatologist if necessary)
• the impact of the disease on the person’s physical, psychological and social wellbeing
• the benefits versus the risks of continued treatment
• the views of the person undergoing treatment (and their family or carers where appropriate). - Monitor people using systemic treatment for all types of psoriasis in accordance with national and local drug guidelines and policy. Take appropriate action in the event of laboratory abnormalities or adverse events.
- Offer adjunctive topical therapy to people with psoriasis using systemic therapy to optimise treatment outcomes.
Offer people with psoriasis who are starting treatment with a systemic non-biological or biological drug the opportunity to participate in longterm safety registries (for example, the British Association of Dermatologists Biologic Interventions Register).
Systemic non-biological therapy
- Offer systemic non-biological therapy to people with any type of psoriasis if:
• it cannot be controlled with topical therapy and
• it has a significant impact on physical, psychological or social wellbeing and
• 1 or more of the following apply:
- psoriasis is extensive (for example, more than 10% of body surface area affected or a PASI score of more than 10) or
- psoriasis is localised and associated with significant functional impairment and/or high levels of distress (for example, severe nail disease or involvement at high-impact sites) or
- phototherapy has been ineffective, cannot be used or has resulted in rapid relapse (rapid relapse is defined as greater than 50% of baseline disease severity within 3 months).
Choice of drugs
- Offer methotrexate as the first choice of systemic agent for people with psoriasis who fulfil the criteria for systemic therapy (off label use in children and young people).
NHS Somerset classify Methotrexate as an amber drug as per Traffic light guidance. See DMARD Shared Care Protocol: Immunomodulatory therapies in rheumatology/gastroenterology and dermatology conditions.
In autoimmune conditions and some cancer therapies, methotrexate should be taken once a week; however, we continue to receive reports of inadvertent overdose due to more frequent dosing (including daily administration). New measures have been implemented to prompt healthcare professionals to record the day of the week for intake and to remind patients of the dosing schedule and the risks of overdose. See Methotrexate once-weekly for autoimmune diseases: new measures to reduce risk of fatal overdose due to inadvertent daily instead of weekly dosing (MHRA September 2020)
Prescribe methotrexate ONCE weekly
Only prescribe 2.5mg tablets (not 10mg)
Folic acid tablets must be prescribed along side methotrexate to limit gastrointestinal and haematological toxicity; 5mg once weekly but not on the same day as methotrexate
See methotrexate patient information booklet (British Association of Dermatologists March 2020)
- In people with both active psoriatic arthritis and any type of psoriasis that fulfils the criteria for systemic therapy consider the choice of systemic agent in consultation with a rheumatologist.
- Offer ciclosporin as the first choice of systemic agent for people who fulfil the criteria for systemic therapy and who:
• need rapid or short-term disease control (for example, a psoriasis flare) or
• have palmoplantar pustulosis or
• are considering conception (both men and women) and systemic therapy cannot be avoided.
(off label use in children and young people).
NHS Somerset classify Ciclosporin as a red drug as per Traffic light guidance.
- Consider changing from methotrexate to ciclosporin (or vice versa) when response to the first-choice systemic treatment is inadequate.
- Consider acitretin for adults, and in exceptional cases only for children and young people, in the following circumstances:
• if methotrexate and ciclosporin are not appropriate or have failed or
• for people with pustular forms of psoriasis.
NHS Somerset classify Acitretin as a red drug as per Traffic light guidance.
Published guidance about the use of remote consultations for pregnancy prevention in women of childbearing potential and monitoring for signs of psychiatric reactions (especially depression) and other safety risks in all patients taking oral retinoid medicines during the COVID-19 pandemic. See Oral retinoid medicines (isotretinoin, alitretinoin, and acitretin): temporary monitoring advice during coronavirus (COVID-19) pandemic (MHRA July 2021)
New prescriber checklists, patient reminder cards, and pharmacy checklists are available to support the Pregnancy Prevention Programme in women taking acitretin, alitretinoin, and isotretinoin. Advice about the risk of neuropsychiatric reactions has been made consistent for all oral retinoid medicines. See Oral retinoid medicines: revised and simplified pregnancy prevention educational materials for healthcare professionals and women (MHRA June 2019)
The risk of foetal malformation with oral retinoids is extremely high, even when used at a low dose or for a short time during pregnancy. All oral retinoids have an associated Pregnancy Prevention Programme (PPP), which is supported by educational material for prescribers, pharmacists, and patients. Women of child-bearing potential should have pregnancy excluded before starting treatment. While taking these medicines, one—or preferably two—different forms of contraception must be consistently used. See Oral retinoids: pregnancy prevention—reminder of measures to minimise teratogenic risk (MHRA June 2013, updated December 2014)
NHS Somerset classify Apremilast as a red drug as per Traffic light guidance.
There is an increased risk that some patients may experience psychiatric symptoms with apremilast, including depression and suicidal thoughts. Stop treatment if patients have new psychiatric symptoms or if existing symptoms worsen. See Apremilast: risk of suicidal thoughts and behaviour (MHRA January 2017)
NHS Somerset classify Dimethyl Fumerate as a red drug as per Traffic light guidance.
The monitoring requirements and discontinuation criteria for dimethyl fumarate have been strengthened following a small number of reports of progressive multifocal leukoencephalopathy (PML) in patients with mild lymphopenia. Continue to monitor lymphocyte counts and advise patients to seek urgent medical attention if they experience any symptoms or signs suggestive of PML. See Dimethyl fumarate: updated advice on the risk of progressive multifocal leukoencephalopathy (PML) associated with mild lymphopenia (MHRA January 2021)
Methotrexate and risk of hepatotoxicity
- When considering the risks and benefits of treating any type of psoriasis with methotrexate, be aware that methotrexate can cause a clinically significant rise in transaminases and that long-term therapy may be associated with liver fibrosis.
- Before and during methotrexate treatment, offer the person with any type of psoriasis an evaluation for potential risk of hepatotoxicity. Use standard liver function tests and serial serum procollagen III levels to monitor for abnormalities during treatment with methotrexate, taking into account pre-existing risk factors (for example, obesity, diabetes and alcohol use), baseline results and trends over time.
- When using serum procollagen III levels to exclude liver fibrosis or cirrhosis, be aware that the:
• test cannot be used in children and young people
• results may be unreliable in people with psoriatic arthritis
• estimated positive predictive value is 23% to 95% and the estimated negative predictive value is 89% to 100%. - Provide advice on modifiable risk factors for liver disease prior to and during therapy, including alcohol intake and weight reduction if appropriate in line with the NICE guidelines on alcohol-use disorders: prevention and obesity prevention. For further advice on how to support attitude and behavioural change, see the NICE guideline on behaviour change.
- Seek timely specialist advice and consider referral to a clinician with expertise in liver disease if the results of liver tests are abnormal.
Systemic biological therapy
- Bological agents for psoriasis should be initiated and supervised only by specialist physicians experienced in the diagnosis and treatment of psoriasis.
NHS Somerset classify Adalimumab, Bimekizumab, Brodalumab, Certolizumab pegol, Etanercept, Guselkumab, Ixekizumab, Risankizumab, Secukinumab, Tildrakizumab and Ustekinumab as red drugs as per Traffic light guidance.
Risk of tuberculosis – screen all patients before starting treatment and monitor them closely. See Tumour necrosis factor alpha inhibitors (MHRA April 2014, updated December 2014)
If you suspect exfoliative dermatitis caused by an adverse drug reaction to ustekinumab, stop treatment. See Ustekinumab: risk of exfoliative dermatitis (MHRA January 2015)
| Therapeutic Area | Formulary Choices | Cost for 28 (unless otherwise stated) | Rationale for decision / comments |
|---|---|---|---|
| Vitamin D analogues | |||
| Calcitriol as Silkis® | 3mcg/g ointment: £18.06 (100g) | Mild to moderately severe psoriasis vulgaris. Adults should apply to affected areas twice per day, once in the morning and once in the evening before retiring and after washing. It is recommended that not more than 35% of the body surface be exposed to daily treatment. Recommended courses of up to 6 weeks. |
|
| Calcipotriol as Dovonex® | 50mcg/g ointment: £7.87 (30g) | Mild to moderately severe psoriasis vulgaris. Adults should apply to the affected area once or twice daily. For maximum benefit use the ointment twice daily. Recommended courses of up to 4-6 weeks. | |
| Vitamin D analogues with corticosteroid | |||
| Calcipotriol and betamethasone | 50mcg/g and 0.5mg/g ointment: £19.84 (30g £18.36 (60g) £33.88 (120g) | Stable psoriasis vulgaris. Adults should apply to the affected area once daily. The body surface area treated with calcipotriol containing medicinal products should not exceed 30%. The recommended treatment period is 4 weeks. | |
| as Wynzora® | 50mcg/g and 0.5mg/g cream: £35.66 (60g) | Mild to moderate psoriasis vulgaris , including scalp. Adults should apply to the affected area once daily. The body surface area treated with calcipotriol containing medicinal products should not exceed 30%. The recommended treatment period is 8 weeks. | |
| gel as generic | 50mcg/g and 0.5mg/g gel: £27.44 (60g) £65.23 (120g) | Psoriasis vulgaris for scalp and non-scalp areas. The recommended treatment period is 4 weeks for scalp areas and 8 weeks for “non-scalp” areas. |
|
| as Enstilar® | 50mcg/g and 0.5mg/g cutaneous foam £39.68 (60g) | Psoriasis vulgaris. Enstilar foam should be applied to the affected area once daily. The recommended treatment period is 4 weeks for flare treatment. For those who have responded are suitable for long-term maintenance treatment and should be applied twice weekly on two non-consecutive days. Between applications there should be 2-3 days without Enstilar treatment. | |
| Tars and Salicylic acid | |||
| as Neutrogena T/Gel® | 2% shampoo £4.36 (125ml) £6.61 (250ml) | Seborrhoeic dermatitis of the scalp, dandruff and scalp psoriasis. Should be used two to three times weekly for 6 weeks. | |
| as Psoriderm® | 2.5% shampoo: £4.74 (250ml) | Psoriasis of the scalp. Use once or twice a week. | |
| 6% cream: £9.42 (225ml) | Sub-acute and chronic psoriasis, including psoriasis of the scalp and flexures. Apply to the affected area once or twice daily. | ||
| Coal tar, salicylic acid and coconut oil. as Capasal® | 1%, 0.5% and 1% shampoo: £4.69 (250ml) | Seborrhoeic eczema, seborrhoeic dermatitis, dandruff and psoriasis. Use once or twice a week. | |
| Coal tar, sulfur and salicylic acid. as Sebco® | 12%, 4% and 2% ointment: £9.42 (40g) £14.88 (100g) | Psoriasis, eczema, seborrhoeic dermatitis and dandruff. Use daily for 3 to 7 days. | |
| as Cocois® | 12%, 4% and 2% ointment: £9.35 (40g) £14.50 (100g) |
||
| Salicylic acid | 2% ointment: £12.49 (450g) | Hyperkeratotic and scaling conditions such as psoriasis. Apply twice daily to the affected area. | |